The Partnership for Research on Ebola Virus in Liberia (PREVAIL)
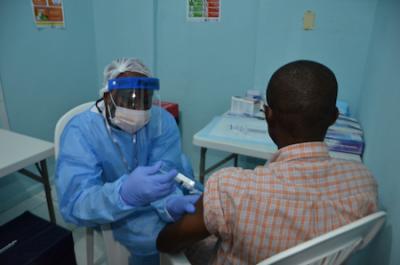
Feb. 2, 2015: A volunteer receives an injection on the first day of the PREVAIL 1 Ebola vaccine clinical trial in Liberia.
The 2014-2016 Ebola outbreak in West Africa was the largest in history, with nearly 28,700 cases and more than 11,300 deaths. As local and international healthcare workers responded to the outbreak, the U.S. National Institute of Allergy and Infectious Diseases (NIAID) and the Ministry of Health in Liberia—one of the hardest-hit countries—established PREVAIL, or the Partnership for Research on Ebola Virus in Liberia. Specifically, the partnership was quickly formed in 2014 in response to a request from the Liberian Minister of Health to the U.S. Secretary of Health and Human Services. Since then PREVAIL has conducted biomedical research according to rigorous international standards to advance Ebola science and to improve the health of people in Liberia and around the world. Collaborative teams of Liberian and NIAID investigators have launched multiple clinical studies to evaluate candidate Ebola treatments and vaccines, and to better understand the long-term health effects of Ebola in those who survive the disease. The PREVAIL clinical research agenda is also expanding beyond Ebola research to address additional health needs of the Liberian people.
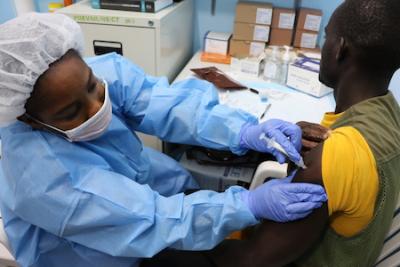
April 3, 2017: Study volunteer receives inoculation at Redemption Hospital in Monrovia on the opening day in Liberia of PREVAC, a Phase 2 Ebola vaccine trial in West Africa.
In February 2015 during the outbreak, PREVAIL launched a randomized, placebo-controlled trial (PREVAIL 1) to assess the safety and efficacy of two experimental vaccines against Ebola—cAd3-EBOZ, developed by NIAID, and Merck’s rVSV-ZEBOV candidate vaccine, developed by the Public Health Agency of Canada. The Phase 2 trial quickly enrolled 1,500 adults 18 years and older. Results published in October 2017 indicate that the two vaccines pose no major safety concerns and can elicit immune responses by one month after initial vaccination that last for at least one year. A long-term follow-up study of the participants continues.
Another randomized, placebo-controlled vaccine trial, known as PREVAIL 5 or PREVAC (Partnership for Research on Ebola VACcination, an international consortium that includes PREVAIL), launched in April 2017 in Guinea and Liberia. A year later, sites in Sierra Leone and Mali opened as well. The trial is evaluating three vaccination strategies to see which regimens hold the most promise in protecting people from Ebola virus disease. The strategies include the following experimental vaccines: Janssen’s Ad26.ZEBOV, Bavarian Nordic’s MVA-BN-Filo (used as a booster), and Merck’s rVSV-ZEBOV. Importantly, this study is enrolling children as young as age 1. Very young children are among the most vulnerable to Ebola infection.
PREVAIL Ebola Treatment Studies
PREVAIL launched a multicenter treatment trial (PREVAIL 2) in March 2015 in the U.S. and West Africa to evaluate Mapp Biopharmaceutical’s antibody-based Ebola treatment ZMapp. Final results reported in October 2016 indicate that the treatment is safe and well-tolerated, and showed a trend toward benefit, but because of the waning Ebola epidemic, the study enrolled too few people to determine definitively whether it is a better treatment for Ebola virus disease than the best available standard of care alone.
Evidence shows that Ebola virus genetical material, or RNA, can persist in semen and other parts of the body after a person has cleared the infection. Therefore, PREVAIL initiated a study (PREVAIL 4) examining whether the experimental drug GS-5734, manufactured by Gilead Sciences, Inc., can eliminate Ebola viral RNA from semen in male survivors. The trial began in July 2016 and expanded to Guinea in 2018.
PREVAIL Studies of Ebola Survivors

A PREVAIL investigator collects a blood sample from a participant in a genomic study, PREVAIL 6, to better understand Ebola outcomes.
Soon after the outbreak waned in Liberia, PREVAIL began a large study of people who have survived Ebola virus disease (EVD). In June 2015, investigators launched PREVAIL 3, a trial following Ebola survivors of any age and their close household and sexual contacts to better understand the long-term health consequences of EVD, determine if survivors develop immunity that could protect them from future Ebola infection, and assess whether previously infected individuals can transmit Ebola to close contacts and sexual partners. Preliminary findings show survivors and close contact controls self-reported a relatively high burden of symptoms. However, certain reported symptoms, such as musculoskeletal pain, were more common among survivors. Findings from physical examinations by clinicians show that abnormalities were much less common than reported symptoms for both survivors and close contacts. However certain findings, such as uveitis (eye inflammation), were observed more commonly in survivors than close contact controls.
Overall, people who contracted Ebola during the recent outbreak had varying responses to infection, with some patients reporting no symptoms and others with fatal infections. In addition, some people rapidly cleared the infection while other patients remained in treatment for weeks or have lingering traces of virus months or years after infection. PREVAIL began a large genomic study, PREVAIL 6, to examine how a person’s genes might affect their response to Ebola. The trial, which began in November 2017, is examining whether certain genes are associated with a higher risk of contracting EVD or of dying from Ebola infection. They also hope to determine if genes influence the problems some Ebola survivors continue to have after recovery, such as uveitis (eye inflammation), or traces of virus lingering in bodily fluids. More than 4,500 participants, including Ebola survivors and close contacts, have enrolled.
PREVAIL Study of Ebola Virus in Eye Tissues and Cataract Surgery
The PREVAIL team launched a small study in September 2017 (PREVAIL 7) to evaluate the safety of removing cataracts and the persistence of pieces of Ebola virus in the eyes. The study identified 37 survivors and contacts with cataracts; of these, 34 underwent surgery to remove the cataract. Cataract is the leading cause of reversible blindness in the world, and surgery is one of the most cost-effective surgical interventions worldwide. The study evaluated the outcomes of this surgery in Ebola survivors, and preliminary results are promising. In addition, the study is analyzing cataract tissue removed at surgery to determine if the Ebola virus can persist in the eyes of survivors after resolution of the acute illness.
Ebola Research in the Democratic Republic of the Congo
NIAID is also conducting Ebola research in the Democratic Republic of the Congo (DRC), where the virus was first discovered.
In August 2018, the DRC Ministry of Health declared the country’s 10th outbreak of Ebola virus disease. In November 2018, NIAID and the National Institute for Biomedical Research (INRB), part of the Democratic Republic of the Congo Ministry of Health, began a Phase 2/3 clinical trial testing multiple investigational Ebola therapies. The trial, known as PALM (short for “Pamoja Tulinde Maisha” a Swahili phrase which roughly translates to “together save lives”), enrolled patients with confirmed Ebola virus disease at Ebola treatment units run by medical humanitarian organizations.
The trial was designed to compare mortality among patients who receive one of three investigational Ebola drugs with a control group of patients who receive the investigational monoclonal antibody cocktail treatment ZMapp, developed by Mapp Biopharmaceutical, Inc. The therapies being tested include: mAb114, a single monoclonal antibody developed by NIAID, with support from the INRB; remdesivir (also known as GS-5734), an antiviral drug developed by Gilead Sciences, Inc., and REGN-EB3 (also known as REGN3470-3471-3479), a monoclonal antibody cocktail developed by Regeneron Pharmaceuticals, Inc.
The trial enrolled 681 people from November 2018 through August 2019. Preliminary evidence indicates that the investigational treatments mAb114 and REGN-EB3 offer patients an increased chance of survival from Ebola compared to ZMapp. Therefore, all Ebola patients at treatment centers in the DRC are now being offered mAb114 and REGN-EB3, including those being enrolled in an ongoing extension phase of the trial.
Scientific Advances