Seasonal influenza vaccines are recommended for everyone aged six months and older as the best way to prevent serious illness or death from this common respiratory disease. But flu vaccines, which elicit protective antibodies aimed at the viral protein hemagglutinin (HA), must be reformulated and readministered every year. This burdensome task is necessary because mutations in HA can lead to a mismatch between the strains of flu virus targeted by a given year’s vaccines and the predominant circulating virus strains. If vaccine-induced antibodies do not fully recognize changed HA in the circulating strains, they may not provide optimal defense against infection. A flu vaccine with a heightened ability to elicit “cross-reactive” antibodies—those able to recognize and bind to changed HA in circulating strains—would likely provide better protection.
Recently, NIAID researchers led by Audray Harris, Ph.D., took a step toward such improved seasonal flu vaccines by using electron microscopy (EM) and a related technology called cryo-electron tomography to study the structures of HA complexes in four commercially available influenza vaccines. “With EM and cryo-EM, we can directly visualize the arrangement of the peanut-shaped HA molecules in each vaccine,” says Dr. Harris. “We wanted to see if there were structural differences and investigate whether any detected differences could be correlated with differing immune responses following vaccination.”
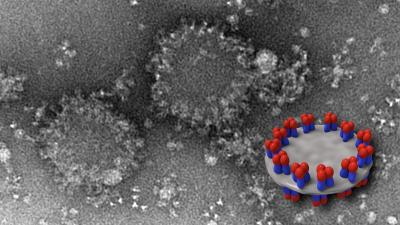
Imaging revealed that the specific shapes, mixtures and abundance of distinct HA-complex structures varied among the vaccines. For example, two vaccines, Flublok and FluzoneHD, contained differing proportions of starfish-shaped structures in which several HA molecules radiated out from a central core. One vaccine, Fluad, contained an HA complex structure not seen in the others. Dubbed “spiked nanodiscs” by the researchers, these newly described structures have up to 50 HA molecules arranged around the periphery of two parallel plate-like central discs. The spikes display both the rounded head and the stalk-like stem of the HA molecule. The HA stem is less prone to change than the head and numerous next-generation candidate flu vaccines are designed to elicit antibodies directed toward it.
All four commercial vaccines were then administered to young mice that had never previously been exposed to influenza virus. Fluad—the one containing HA complex displayed on the spiked nanodiscs—elicited the highest levels of cross-reactive antibodies. These early-stage animal studies cannot be directly translated to humans, Dr. Harris notes, because, among other reasons, people are typically exposed to flu virus through vaccination or natural exposure very early in life, unlike the influenza-naive mice used in the study. Further studies are now underway in Dr. Harris’s lab to better understand the role played by spiked nanodiscs. It may be, he explains, that the specific arrangement of multiple head-and-stem HA molecules on each disc allows the immune system a less obscured “view” of that vaccine component which, in turns, allows more cross-reactive antibodies to be produced.
Dr. Harris notes that, although they have more work to do to confirm these early findings, “our direct view of the HA complexes in each vaccine gave us an ‘aha’ moment, and we believe these novel nanodiscs may provide a path to improved seasonal flu vaccines.”
Reference:
ML Myers, JR Gallagher, et al. Commercial influenza vaccines vary in HA-complex structure and in induction of cross-reactive HA antibodies. Nature Communications DOI: 10.1038/s41467-023-37162-z (2023).