217 Results
NIH-Sponsored Trial of Lassa Vaccine Opens
March 17, 2025
An NIAID-sponsored clinical trial of an experimental vaccine to prevent Lassa fever has begun.
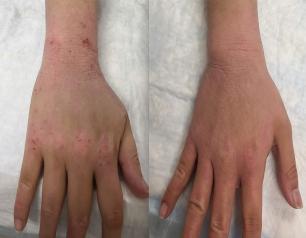
Topical Steroid Withdrawal Diagnostic Criteria Defined by NIH Researchers
March 14, 2025
Topical steroid withdrawal (TSW) results in dermatitis that is distinct from eczema and is caused by an excess of NAD+, an essential chemical compound in the body, according to a new study from NIAID researchers.
Tecovirimat Is Safe but Ineffective as Treatment for Clade II Mpox
March 12, 2025
Monotherapy with the antiviral drug tecovirimat was safe but ineffective as an mpox treatment in an international clinical trial.
Omalizumab Treats Multi-Food Allergy Better than Oral Immunotherapy
March 3, 2025
The high rate of allergic reactions and other intolerable side effects of oral immunotherapy in the NIH-funded trial explained the superiority of omalizumab.

NIH-Funded Clinical Trial Will Evaluate New Dengue Therapeutic
February 11, 2025
A Phase 2 clinical trial will test the safety and efficacy of an experimental treatment for dengue, a viral disease transmitted by mosquitoes.

Single Dose of Broadly Neutralizing Antibody Protects Macaques from H5N1 Influenza
February 11, 2025
A single dose of a broadly neutralizing antibody given prior to virus exposure protects macaques from severe H5N1 avian influenza, NIH scientists report.
Influenza A Viruses Adapt Shape in Response to Environmental Pressures
February 10, 2025
Influenza A virus particles strategically adapt their shape – to become either spheres or larger filaments – to favor their ability to infect cells depending on environmental conditions, a new NIAID study published in Nature Microbiology reveals. This previously unrecognized response could help explain how influenza A and other viruses persist in populations, evade immune responses, and acquire adaptive mutations. The scientists designed the study to determine why many influenza A virus particles exist as filaments, which requires more energy to form than a sphere.
Therapy Helps Peanut-Allergic Kids Tolerate Tablespoons of Peanut Butter
February 10, 2025
Eating slowly increasing amounts of peanut butter enabled 100% of kids with peanut allergy to consume 3 tablespoons of peanut butter without an allergic reaction.

As Prevention Strategy for Sexually Transmitted Infections Rolls Out, Experts Highlight both Promise and Knowledge Gaps
January 6, 2025
DoxyPEP is reducing the rate of syphilis and chlamydia but has had little to no effect on gonorrhea and needs close monitoring for antibiotic resistance.
NIH Researchers Discover Novel Class of Anti-Malaria Antibodies
January 3, 2025
New antibodies that bind to a previously untargeted portion of the malaria parasite could lead to new monoclonal antibody treatments and vaccines for malaria.
NIH Officials Assess Threat of H5N1
December 31, 2024
HPAI H5N1 influenza remains a low risk to most Americans, but that does not diminish concern about the virus, NIAID experts say.
NIH Study Finds Tecovirimat Was Safe but Did Not Improve Mpox Resolution or Pain
December 10, 2024
Tecovirimat was safe but did not reduce the time to lesion resolution or reduce pain among adults with mild to moderate clade II mpox and a low risk of severe disease in an international study.
Single Mutation in H5N1 Influenza Surface Protein Could Enable Easier Human Infection
December 6, 2024
A single modification in the protein found on the surface of the highly pathogenic avian influenza (HPAI) H5N1 influenza virus currently circulating in U.S. dairy cows could allow for easier transmission among humans, according to new research funded by the National Institutes of Health (NIH) and published today in the journal Science. The study results reinforce the need for continued, vigilant surveillance and monitoring of HPAI H5N1 for potential genetic changes that could make the virus more transmissible in humans.
NIH Statement on World AIDS Day
December 2, 2024
Together with our partners, the National Institutes of Health (NIH) commemorates World AIDS Day and affirms our commitment to bolstering the extraordinary gains achieved in HIV science and to persevering until we end HIV-related illness and stigma. As we mark this observance, we celebrate the people who enable scientific progress, honor the loved ones and leaders we have lost, and reflect on the work that remains to ensure the health and life quality of all people affected by, and living with, HIV.
NIH Trial of Rectal Microbicide for HIV Prevention Begins in the United States
October 31, 2024
A clinical trial has launched to examine the safety and acceptability of a novel rectal HIV microbicide douche containing the antiretroviral drug tenofovir. While HIV incidence is slowly decreasing in the United States, 67% of U.S. HIV diagnoses from 2018-2022 were among gay, bisexual, and other men who have sex with men, pointing to the need for expanded HIV prevention options. The mid-stage study is sponsored by the National Institutes of Health’s National Institute of Allergy and Infectious Diseases and implemented through the NIH-funded HIV Prevention Trials Network.
Bovine H5N1 Influenza from Infected Worker Transmissible and Lethal in Animal Models
October 28, 2024
Bovine H5N1 influenza virus taken from eye of infected worker transmissible and lethal in animal models.
Kidney Transplantation Between Donors and Recipients with HIV Is Safe
October 16, 2024
Kidney transplantation from deceased donors with HIV to recipients with HIV was safe and comparable to kidney transplantation from donors without HIV.
Mpox Vaccine Is Safe and Generates a Robust Antibody Response in Adolescents
October 16, 2024
A clinical trial of an mpox vaccine in adolescents found it was safe and generated an antibody response equivalent to that seen in adults. Results were presented at IDWeek2024.
NIAID Selects Sarah Read as Principal Deputy Director
October 2, 2024
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), has named Sarah W. Read, M.D., MHS, as the principal deputy director for the institute.

NIH Releases Mpox Research Agenda
September 17, 2024
The NIAID mpox research agenda focuses on four key objectives: increasing knowledge about the biology of all clades—also known as strains—of the virus that causes mpox, including how the virus is transmitted and how people’s immune systems respond to it; evaluating dosing regimens of current vaccines to stretch the vaccine supply and developing novel vaccine concepts; advancing existing and novel treatments, including antivirals and monoclonal antibodies; and supporting strategies for detecting the virus to facilitate clinical care and epidemiological surveillance.
NIH Awards Establish Pandemic Preparedness Research Network
September 13, 2024
The Research and Development of Vaccines and Monoclonal Antibodies for Pandemic Preparedness network—called ReVAMPP—will focus its research efforts on “prototype pathogens,” representative pathogens from virus families known to infect humans, and high-priority pathogens that have the potential to cause deadly diseases. The pandemic preparedness research network will conduct research on high-priority pathogens most likely to threaten human health with the goal of developing effective vaccines and monoclonal antibodies.
Emergency Department Screening More Than Doubles Detection of Syphilis Cases
September 10, 2024
Providing optional syphilis tests to most people seeking care at a large emergency department led to a dramatic increase in syphilis screening and diagnosis, according to study of nearly 300,000 emergency department encounters in Chicago. Most people diagnosed had no symptoms, which suggests that symptom-based testing strategies alone could miss opportunities to diagnose and treat people with syphilis.
NIH Awards Will Support Innovation in Syphilis Diagnostics
September 3, 2024
NIAID has awarded grants for 10 projects to improve diagnostic tools for congenital and adult syphilis—conditions currently diagnosed with a sequence of tests, each with limited precision. The Centers for Disease Control and Prevention estimates that adult and congenital syphilis cases increased by 80% and 183% respectively between 2018 and 2022—a crisis that prompted the U.S. Department of Health and Human Services (HHS) to establish a national taskforce to respond to the epidemic.
The Antiviral Tecovirimat is Safe but Did Not Improve Clade I Mpox Resolution in Democratic Republic of the Congo
August 15, 2024
The antiviral drug tecovirimat did not reduce the duration of mpox lesions among children and adults with clade I mpox in the Democratic Republic of the Congo (DRC), based on an initial analysis of data from a randomized, placebo-controlled trial.
Candidate Malaria Vaccine Provides Lasting Protection in NIH-Sponsored Trials
August 14, 2024
Two National Institutes of Health (NIH)-supported trials of an experimental malaria vaccine in healthy Malian adults found that all three tested regimens were safe.