9 Results
NIH Researchers Discover Novel Class of Anti-Malaria Antibodies
January 3, 2025
New antibodies that bind to a previously untargeted portion of the malaria parasite could lead to new monoclonal antibody treatments and vaccines for malaria.
Candidate Malaria Vaccine Provides Lasting Protection in NIH-Sponsored Trials
August 14, 2024
Two National Institutes of Health (NIH)-supported trials of an experimental malaria vaccine in healthy Malian adults found that all three tested regimens were safe.
Experimental NIH Malaria Monoclonal Antibody Protective in Malian Children
April 26, 2024
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
NIAID Marks World Malaria Day
April 25, 2023
World Malaria Day is an opportunity to reflect on continuing challenges posed by malaria and reaffirm a commitment to overcoming them. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, joins with the global health community in recognizing this year’s theme of “Time to Deliver on Zero Malaria: Invest, Innovate, Implement.”
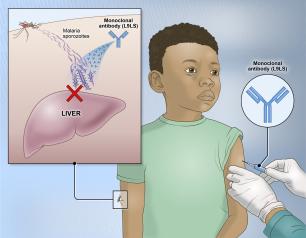
Monoclonal Antibody Prevents Malaria Infection in African Adults
October 31, 2022
One dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Mali, Africa, a National Institutes of Health clinical trial has found. The antibody was up to 88.2% effective at preventing infection over a 24-week period, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in an endemic region.
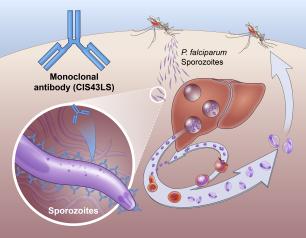
Monoclonal Antibody Prevents Malaria in U.S. Adults, NIH Trial Shows
August 4, 2022
One injection of a candidate monoclonal antibody (mAb) known as L9LS was found to be safe and highly protective in U.S. adults exposed to the malaria parasite, according to results from a National Institutes of Health Phase 1 clinical trial published in The New England Journal of Medicine.
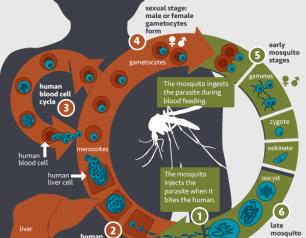
NIAID Marks World Malaria Day
April 25, 2022
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), reaffirms our commitment to developing safe and effective medical tools against malaria, and, in the words of this year’s World Malaria Day theme, “Harnessing innovation to reducing the malaria disease burden and saving lives.”
Monoclonal Antibody Prevents Malaria in Small NIH Trial
August 11, 2021
One dose of a new monoclonal antibody discovered and developed at the National Institutes of Health safely prevented malaria for up to nine months in people who were exposed to the malaria parasite. The small, carefully monitored clinical trial is the first to demonstrate that a monoclonal antibody can prevent malaria in people. The trial was sponsored and conducted by scientists from the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH, and was funded by NIAID.

Investigational Malaria Vaccine Gives Strong, Lasting Protection
June 30, 2021
Two U.S. Phase 1 clinical trials of a novel candidate malaria vaccine have found that the regimen conferred unprecedentedly high levels of durable protection when volunteers were later exposed to disease-causing malaria parasites. The vaccine combines live parasites with either of two widely used antimalarial drugs—an approach termed chemoprophylaxis vaccination. A Phase 2 clinical trial of the vaccine is now underway in Mali, a malaria-endemic country.


