18 Results
Single Dose of Broadly Neutralizing Antibody Protects Macaques from H5N1 Influenza
February 11, 2025
A single dose of a broadly neutralizing antibody given prior to virus exposure protects macaques from severe H5N1 avian influenza, NIH scientists report.
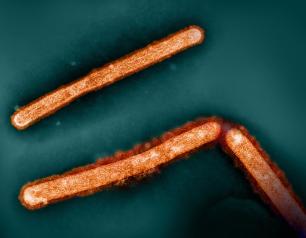
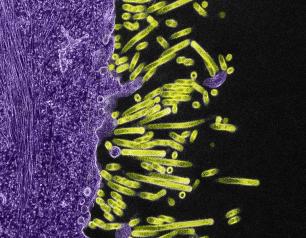
Influenza A Viruses Adapt Shape in Response to Environmental Pressures
February 10, 2025
Influenza A virus particles strategically adapt their shape – to become either spheres or larger filaments – to favor their ability to infect cells depending on environmental conditions, a new NIAID study published in Nature Microbiology reveals. This previously unrecognized response could help explain how influenza A and other viruses persist in populations, evade immune responses, and acquire adaptive mutations. The scientists designed the study to determine why many influenza A virus particles exist as filaments, which requires more energy to form than a sphere.
NIH Officials Assess Threat of H5N1
December 31, 2024
HPAI H5N1 influenza remains a low risk to most Americans, but that does not diminish concern about the virus, NIAID experts say.
Single Mutation in H5N1 Influenza Surface Protein Could Enable Easier Human Infection
December 6, 2024
A single modification in the protein found on the surface of the highly pathogenic avian influenza (HPAI) H5N1 influenza virus currently circulating in U.S. dairy cows could allow for easier transmission among humans, according to new research funded by the National Institutes of Health (NIH) and published today in the journal Science. The study results reinforce the need for continued, vigilant surveillance and monitoring of HPAI H5N1 for potential genetic changes that could make the virus more transmissible in humans.
Bovine H5N1 Influenza from Infected Worker Transmissible and Lethal in Animal Models
October 28, 2024
Bovine H5N1 influenza virus taken from eye of infected worker transmissible and lethal in animal models.
Features of H5N1 Influenza Viruses in Dairy Cows May Facilitate Infection, Transmission in Mammals
July 8, 2024
A series of experiments with highly pathogenic H5N1 avian influenza (HPAI H5N1) viruses circulating in infected U.S. dairy cattle found that viruses derived from lactating dairy cattle induced severe disease in mice and ferrets when administered via intranasal inoculation.
Infectious H5N1 Influenza Virus in Raw Milk Rapidly Declines with Heat Treatment
June 14, 2024
The amount of infectious H5N1 influenza viruses in raw milk rapidly declined with heat treatment in laboratory research conducted by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. However, small, detectable amounts of infectious virus remained in raw milk samples with high virus levels when treated at 72 degrees Celsius (161.6 degrees Fahrenheit) for 15 seconds—one of the standard pasteurization methods used by the dairy industry.

NIH Releases H5N1 Influenza Research Agenda
June 5, 2024
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has released its plan for advancing H5N1 influenza basic research and translating those findings into strategies and interventions that can benefit people. The research agenda focuses on four key objectives: increasing understanding of the biology of H5N1 viruses and the factors that influence their ability to transmit and cause disease; developing and evaluating prevention strategies, such as vaccines; advancing existing and novel treatments, including antivirals and monoclonal antibodies; and supporting strategies for detecting H5N1 virus. The NIAID Research Agenda for 2024 H5N1 Influenza – May 2024 aligns with the NIAID role in the federal public health response to the U.S. outbreak of H5N1 influenza in people, dairy cows and other animals.
High H5N1 Influenza Levels Found in Mice Given Raw Milk from Infected Dairy Cows
May 24, 2024
Mice administered raw milk samples from dairy cows infected with H5N1 influenza experienced high virus levels in their respiratory organs and lower virus levels in other vital organs, according to findings published in the New England Journal of Medicine. The results suggest that consumption of raw milk by animals poses a risk for H5N1 infection and raises questions about its potential risk in humans.

New Antibodies Target “Dark Side” of Influenza Virus Protein
March 1, 2024
Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored “dark side” of the neuraminidase (NA) protein head. The antibodies target a region of the NA protein that is common among many influenza viruses, including H3N2 subtype viruses, and could be a new target for countermeasures. The research, led by scientists at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center, part of NIH, was published today in Immunity.
NIH Clinical Trial of Universal Flu Vaccine Candidate Begins
September 15, 2023
Enrollment in a Phase 1 trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland. The trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, and will evaluate the investigational vaccine for safety and its ability to elicit an immune response.
Clinical Trial of mRNA Universal Influenza Vaccine Candidate Begins
May 15, 2023
A clinical trial of an experimental universal influenza vaccine developed by researchers at the National Institute of Allergy and Infectious Diseases’ (NIAID) Vaccine Research Center (VRC), part of the National Institutes of Health, has begun enrolling volunteers at Duke University in Durham, North Carolina. This Phase 1 trial will test the experimental vaccine, known as H1ssF-3928 mRNA-LNP, for safety and its ability to induce an immune response.
Developing Mucosal Vaccines for Respiratory Viruses
January 11, 2023
Vaccines that provide long-lasting protection against influenza, coronaviruses and respiratory syncytial virus (RSV) have proved exceptionally difficult to develop. In a new review article in Cell Host & Microbe, researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, explore the challenges and outline approaches to improved vaccines. Anthony S. Fauci, M.D., former NIAID director, is an author along with Jeffery K. Taubenberger, M.D., Ph.D., and David M. Morens, M.D.
Trial of Potential Universal Flu Vaccine Opens at NIH Clinical Center
June 28, 2022
A Phase 1 clinical trial of a novel influenza vaccine has begun inoculating healthy adult volunteers at the National Institutes of Health Clinical Center in Bethesda, Maryland. The placebo-controlled trial will test the safety of a candidate vaccine, BPL-1357, and its ability to prompt immune responses.
Leadership Transition at the NIAID Vaccine Research Center
February 16, 2022
Dr. Fauci expresses gratitude to John R. Mascola, M.D., as he announces his retirement as Director of the Dale and Betty Bumpers Vaccine Research Center at the National Institute of Allergy and Infectious Diseases.
NIH Launches Clinical Trial of Universal Influenza Vaccine Candidate
June 1, 2021
A first-in-human, Phase 1 trial assessing the safety and immunogenicity of an investigational nanoparticle influenza vaccine designed to provide long-lasting protection against multiple flu virus strains has begun at the National Institutes of Health Clinical Center in Bethesda, Maryland. Healthy participants 18 to 50 years old will receive either a licensed seasonal influenza vaccine or the experimental vaccine, FluMos-v1.
NIAID Funds New Influenza Research Network
April 14, 2021
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has established a network of research sites to study the natural history, transmission and pathogenesis of influenza and provide an international research infrastructure to address influenza outbreaks.
Intranasal Influenza Vaccine Spurs Strong Immune Response in Phase 1 Study
February 3, 2021
An experimental influenza vaccine was safe and produced a durable immune response when tested in a Phase 1 study, NIH reports.