19 Results
Topical Steroid Withdrawal Diagnostic Criteria Defined by NIH Researchers
March 14, 2025
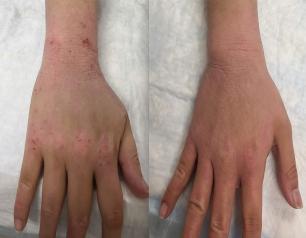
Topical steroid withdrawal (TSW) results in dermatitis that is distinct from eczema and is caused by an excess of NAD+, an essential chemical compound in the body, according to a new study from NIAID researchers.
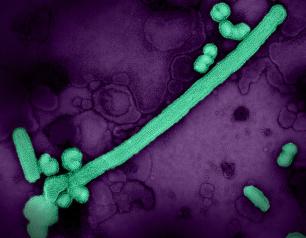
Influenza A Viruses Adapt Shape in Response to Environmental Pressures
February 10, 2025
Influenza A virus particles strategically adapt their shape – to become either spheres or larger filaments – to favor their ability to infect cells depending on environmental conditions, a new NIAID study published in Nature Microbiology reveals. This previously unrecognized response could help explain how influenza A and other viruses persist in populations, evade immune responses, and acquire adaptive mutations. The scientists designed the study to determine why many influenza A virus particles exist as filaments, which requires more energy to form than a sphere.
Kidney Transplantation Between Donors and Recipients with HIV Is Safe
October 16, 2024
Kidney transplantation from deceased donors with HIV to recipients with HIV was safe and comparable to kidney transplantation from donors without HIV.
Candidate Malaria Vaccine Provides Lasting Protection in NIH-Sponsored Trials
August 14, 2024
Two National Institutes of Health (NIH)-supported trials of an experimental malaria vaccine in healthy Malian adults found that all three tested regimens were safe. One of the trials enrolled 300 healthy women ages 18 to 38 years who anticipated becoming pregnant soon after immunization. That trial began with drug treatment to remove malaria parasites, followed by three injections spaced over a month of either saline placebo or the investigational vaccine at one of two dosages.
Existing Drug Shows Promise as Treatment for Rare Genetic Disorder
May 30, 2024
A drug approved to treat certain autoimmune diseases and cancers successfully alleviated symptoms of a rare genetic syndrome called autoimmune polyendocrine syndrome type 1 (APS-1). Researchers identified the treatment based on their discovery that the syndrome is linked to elevated levels of interferon-gamma (IFN-gamma), a protein involved in immune system responses, providing new insights into the role of IFN-gamma in autoimmunity.
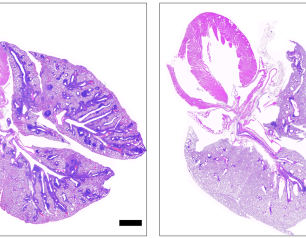
NIH Study Shows Chronic Wasting Disease Unlikely to Move from Animals to People
May 17, 2024
A new study of prion diseases, using a human cerebral organoid model, suggests there is a substantial species barrier preventing transmission of chronic wasting disease (CWD) from cervids—deer, elk and moose—to people. The findings, from National Institutes of Health scientists and published in Emerging Infectious Diseases, are consistent with decades of similar research in animal models at the NIH’s National Institute of Allergy and Infectious Diseases (NIAID).
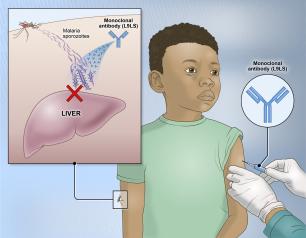
Experimental NIH Malaria Monoclonal Antibody Protective in Malian Children
April 26, 2024
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
NIH Scientists Find Weak Points on Epstein-Barr Virus
March 12, 2024
Studies of interactions between two lab-generated monoclonal antibodies (mAbs) and an essential Epstein-Barr virus (EBV) protein have uncovered targets that could be exploited in designing treatments and vaccines for this extremely common virus. The research was led by Jeffrey I. Cohen, M.D., and colleagues from NIAID.

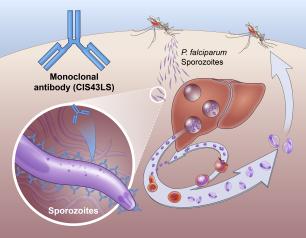
Monoclonal Antibody Prevents Malaria Infection in African Adults
October 31, 2022
One dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Mali, Africa, a National Institutes of Health clinical trial has found. The antibody was up to 88.2% effective at preventing infection over a 24-week period, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in an endemic region.
Trial of Potential Universal Flu Vaccine Opens at NIH Clinical Center
June 28, 2022
A Phase 1 clinical trial of a novel influenza vaccine has begun inoculating healthy adult volunteers at the National Institutes of Health Clinical Center in Bethesda, Maryland. The placebo-controlled trial will test the safety of a candidate vaccine, BPL-1357, and its ability to prompt immune responses. The vaccine candidate was developed by researchers at the National Institute of Allergy and Infectious Diseases (NIAID). The single-site trial can enroll up to 100 people aged 18 to 55 years and is led by NIAID investigator Matthew J. Memoli, M.D.
NIH Launches Trial to Study Allergic Reactions to COVID-19 mRNA Vaccine
March 9, 2022
Researchers from the National Institute of Allergy and Infectious Diseases (NIAID) are conducting a clinical trial designed to help understand rare but potentially serious systemic allergic reactions to COVID-19 mRNA vaccines. The single-site trial will enroll up to 100 people aged 16 to 69 years old who had an allergic reaction to a first dose of COVID-19 mRNA vaccines. Study participants will receive a second dose of vaccine as inpatients under carefully controlled conditions at the NIH Clinical Center in Bethesda, Maryland.
NIH Scientists Urge Pursuit of Universal Coronavirus Vaccine
December 16, 2021
A growing body of scientific evidence, considered together with ecological reality, strongly suggests that novel coronaviruses will continue to infect bats and other animal reservoirs and potentially emerge to pose a pandemic threat to humans. To counter future coronavirus outbreaks the global research community should focus on characterizing the range of coronavirus genetic diversity in multiple animal species; better understanding coronavirus disease pathogenesis in laboratory animal models and people; and applying this knowledge to the development of long-lasting, broadly protective coronavirus vaccines.

Experimental mRNA HIV Vaccine Safe, Shows Promise in Animals
December 9, 2021
An experimental HIV vaccine based on mRNA—the same platform technology used in two highly effective COVID-19 vaccines—shows promise in mice and non-human primates, according to scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. Their results, published in Nature Medicine, show that the novel vaccine was safe and prompted desired antibody and cellular immune responses against an HIV-like virus.
NIAID Scientists Find a Key to Hepatitis C Entry into Cells
September 21, 2021
Understanding Structure of HCV Proteins Could Aid in Vaccine Development
Investigational Malaria Vaccine Gives Strong, Lasting Protection
June 30, 2021
Two U.S. Phase 1 clinical trials of a novel candidate malaria vaccine have found that the regimen conferred unprecedentedly high levels of durable protection when volunteers were later exposed to disease-causing malaria parasites. The vaccine combines live parasites with either of two widely used antimalarial drugs—an approach termed chemoprophylaxis vaccination. A Phase 2 clinical trial of the vaccine is now underway in Mali, a malaria-endemic country.

Gene Therapy Restores Immune Function in Children with Rare Immunodeficiency
May 11, 2021
An investigational gene therapy can safely restore the immune systems of infants and children who have a rare, life-threatening inherited immunodeficiency disorder, according to research supported in part by the National Institutes of Health.

COVID-19 Vaccine Responses to be Studied in People with Immune Deficits
April 23, 2021
A study assessing how people with immune system deficiencies or dysregulations respond to COVID-19 vaccination has begun enrolling participants at the National Institutes of Health Clinical Center in Bethesda, Maryland. The single-site study is led by researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and aims to enroll 500 people, 400 with primary or secondary immune system disorders and 100 without such conditions.
T Cells Recognize Recent SARS-CoV-2 Variants
March 30, 2021
When variants of SARS-CoV-2 (the virus that causes COVID-19) emerged in late 2020, concern arose that they might elude protective immune responses generated by prior infection or vaccination, potentially making re-infection more likely or vaccination less effective. To investigate this possibility, researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and colleagues analyzed blood cell samples from 30 people who had contracted and recovered from COVID-19 prior to the emergence of virus variants.
Media Availability—NIH Officials Highlight COVID-19 Vaccine Facts, Unknowns for Healthcare Providers
January 18, 2021
NIAID Director urges healthcare providers to be able to explain the latest data supporting the safety and efficacy of vaccines for COVID-19.

