54 Results
Statement from NIH and BARDA on the FDA Emergency Use Authorization of the Janssen COVID-19 Vaccine
February 27, 2021
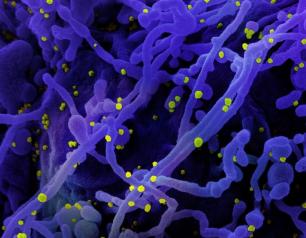
Today, the U.S. Food and Drug Administration issued an Emergency Use Authorization (EUA) to the Janssen Pharmaceuticals Companies of Johnson & Johnson for its single-shot COVID-19 vaccine, called Ad.26.COV2S or JNJ-78436725. The Janssen vaccine is a recombinant vector vaccine that uses a human adenovirus to express the spike protein found on the surface of the SARS-CoV-2 virus that causes COVID-19.
Clinical Trial in Hospitalized COVID-19 Patients Evaluates Long-Acting Antibody Therapy
February 8, 2021
A NIAID clinical trial began evaluating the safety of an investigational long-acting antibody combination for people hospitalized with COVID-19.
Statement—Janssen Investigational COVID-19 Vaccine—Interim Analysis
January 29, 2021
NIH reports that an investigational COVID-19 vaccine by Janssen Pharmaceuticals appears to be safe and effective at preventing COVID-19 in adults.
Media Availability—NIH Officials Highlight COVID-19 Vaccine Facts, Unknowns for Healthcare Providers
January 18, 2021
NIAID Director urges healthcare providers to be able to explain the latest data supporting the safety and efficacy of vaccines for COVID-19.

