37 Results
NIH Scientists Describe “Multi-Kingdom Dialogue” Between Internal, External Microbiota
June 23, 2021
National Institutes of Health scientists and their collaborators have identified an internal communication network in mammals that may regulate tissue repair and inflammation, providing new insights on how diseases such as obesity and inflammatory skin disorders develop.
Experimental Antiviral for COVID-19 Effective in Hamster Study
April 16, 2021
The experimental antiviral drug MK-4482 significantly decreased levels of virus and disease damage in the lungs of hamsters treated for SARS-CoV-2 infection, according to a new study from National Institutes of Health scientists. SARS-CoV-2 is the virus that causes COVID-19. MK-4482, delivered orally, is now in human clinical trials. Remdesivir, an antiviral drug already approved by the U.S. Food and Drug Administration for use against COVID-19, must be provided intravenously, making its use primarily limited to clinical settings.
NIAID Issues New Awards to Fund “Pan-Coronavirus” Vaccines
September 28, 2021
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has awarded approximately $36.3 million to three academic institutions to conduct research to develop vaccines to protect against multiple types of coronaviruses and viral variants. The awards are intended to fuel vaccine research for a diverse family of coronaviruses, with a primary focus on potential pandemic-causing coronaviruses, such as SARS-CoV-2.
NIH Scientists Find that Salmonella Use Intestinal Epithelial Cells to Colonize the Gut
May 26, 2021
The immune system’s attempt to eliminate Salmonella bacteria from the gastrointestinal (GI) tract instead facilitates colonization of the intestinal tract and fecal shedding, according to National Institutes of Health scientists. The study, published in Cell Host & Microbe, was conducted by National Institute of Allergy and Infectious Diseases (NIAID) scientists at Rocky Mountain Laboratories in Hamilton, Montana.

T Cells Recognize Recent SARS-CoV-2 Variants
March 30, 2021
When variants of SARS-CoV-2 (the virus that causes COVID-19) emerged in late 2020, concern arose that they might elude protective immune responses generated by prior infection or vaccination, potentially making re-infection more likely or vaccination less effective. To investigate this possibility, researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and colleagues analyzed blood cell samples from 30 people who had contracted and recovered from COVID-19 prior to the emergence of virus variants.
Scientists Identify Locations of Early Prion Protein Deposition in Retina
January 29, 2021
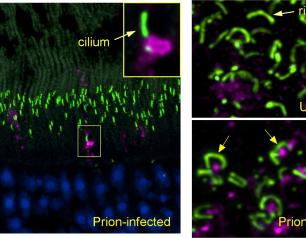
The earliest eye damage from prion disease takes place in the cone photoreceptor cells according to a new NIH study of prion protein accumulation.
Mouse Study Shows Bacteriophage Therapy Could Fight Drug-Resistant Klebsiella pneumoniae
February 23, 2021
NIH study finds using viruses instead of antibiotics to tame drug-resistant bacteria is a promising strategy, known as bacteriophage or “phage therapy.”
COVID-19 Vaccine Responses to be Studied in People with Immune Deficits
April 23, 2021
A study assessing how people with immune system deficiencies or dysregulations respond to COVID-19 vaccination has begun enrolling participants at the National Institutes of Health Clinical Center in Bethesda, Maryland. The single-site study is led by researchers from the National Institute of Allergy and Infectious Diseases (NIAID) and aims to enroll 500 people, 400 with primary or secondary immune system disorders and 100 without such conditions.
NIH Launches Clinical Trial of Universal Influenza Vaccine Candidate
June 1, 2021
A first-in-human, Phase 1 trial assessing the safety and immunogenicity of an investigational nanoparticle influenza vaccine designed to provide long-lasting protection against multiple flu virus strains has begun at the National Institutes of Health Clinical Center in Bethesda, Maryland. Healthy participants 18 to 50 years old will receive either a licensed seasonal influenza vaccine or the experimental vaccine, FluMos-v1.
Adjuvant Developed with NIH Funding Enhances Efficacy of India’s COVID-19 Vaccine
June 29, 2021
An adjuvant developed with funding from the National Institutes of Health has contributed to the success of the highly efficacious COVAXIN COVID-19 vaccine, which roughly 25 million people have received to date in India and elsewhere. Adjuvants are substances formulated as part of a vaccine to boost immune responses and enhance a vaccine’s effectiveness. COVAXIN was developed and is manufactured in India, which is currently suffering a devastating health crisis due to COVID-19.
NIH Awards Grants to Support Bacteriophage Therapy Research
March 11, 2021
NIAID has awarded $2.5 million in grants to 12 institutes around the world to support research on bacteriophage therapy.
Statement—NIH Celebrates FDA Approval of Long-Acting Injectable Drug for HIV Prevention
December 21, 2021
U.S. Food and Drug Administration announced its first approval of a long-acting HIV prevention medication. Developed by ViiV Healthcare, the medicine is long-acting cabotegravir injected once every two months. FDA has approved the medicine for use by adults and adolescents weighing at least 35 kilograms who are at risk of sexually acquiring HIV.

