48 Results
U.S. Clinical Trials Begin for Twice-Yearly HIV Prevention Injection
June 4, 2024
Two clinical trials have launched to examine a novel long-acting form of HIV pre-exposure prophylaxis (PrEP) in cisgender women and people who inject drugs. The mid-stage studies will assess the safety, acceptability, and pharmacokinetics (how a drug moves through the body) of lenacapavir, an antiretroviral drug administered by injection every six months. The studies are sponsored and funded by Gilead Sciences, Inc., and implemented through the HIV Prevention Trails Network (HPTN).
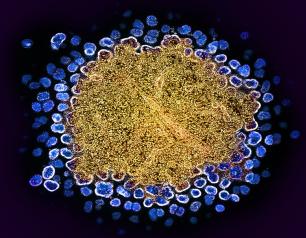
Existing Drug Shows Promise as Treatment for Rare Genetic Disorder
May 30, 2024
A drug approved to treat certain autoimmune diseases and cancers successfully alleviated symptoms of a rare genetic syndrome called autoimmune polyendocrine syndrome type 1 (APS-1). Researchers identified the treatment based on their discovery that the syndrome is linked to elevated levels of interferon-gamma (IFN-gamma), a protein involved in immune system responses, providing new insights into the role of IFN-gamma in autoimmunity.
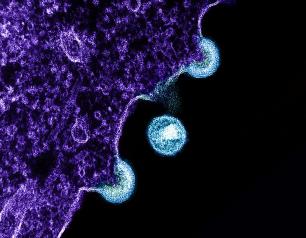
Novel Vaccine Concept Generates Immune Responses that Could Produce Multiple Types of HIV Broadly Neutralizing Antibodies
May 30, 2024
Using a combination of cutting-edge immunologic technologies, researchers have successfully stimulated animals’ immune systems to induce rare precursor B cells of a class of HIV broadly neutralizing antibodies (bNAbs). The findings, published today in Nature Immunology, are an encouraging, incremental step in developing a preventive HIV vaccine.
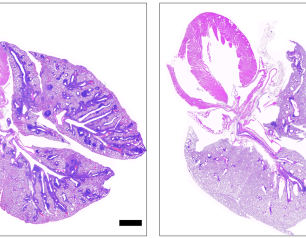
Introducing Peanut in Infancy Prevents Peanut Allergy into Adolescence
May 28, 2024
Feeding children peanut products regularly from infancy to age 5 years reduced the rate of peanut allergy in adolescence by 71%, even when the children ate or avoided peanut products as desired for many years. These new findings, from a study sponsored and co-funded by the National Institutes of Health’s National Institute of Allergy and Infectious Diseases (NIAID), provide conclusive evidence that achieving long-term prevention of peanut allergy is possible through early allergen consumption.

High H5N1 Influenza Levels Found in Mice Given Raw Milk from Infected Dairy Cows
May 24, 2024
Mice administered raw milk samples from dairy cows infected with H5N1 influenza experienced high virus levels in their respiratory organs and lower virus levels in other vital organs, according to findings published in the New England Journal of Medicine. The results suggest that consumption of raw milk by animals poses a risk for H5N1 infection and raises questions about its potential risk in humans.

NIH Study Shows Chronic Wasting Disease Unlikely to Move from Animals to People
May 17, 2024
A new study of prion diseases, using a human cerebral organoid model, suggests there is a substantial species barrier preventing transmission of chronic wasting disease (CWD) from cervids—deer, elk and moose—to people. The findings, from National Institutes of Health scientists and published in Emerging Infectious Diseases, are consistent with decades of similar research in animal models at the NIH’s National Institute of Allergy and Infectious Diseases (NIAID).
Lower Dose of Mpox Vaccine Is Safe and Generates Six-Week Antibody Response Equivalent to Standard Regimen
April 27, 2024
A dose-sparing intradermal mpox vaccination regimen was safe and generated an antibody response equivalent to that induced by the standard regimen at six weeks (two weeks after the second dose), according to findings presented today at the European Society of Clinical Microbiology and Infectious Diseases Global Congress in Barcelona. The results suggest that antibody responses contributed to the effectiveness of dose-sparing mpox vaccine regimens used during the 2022 U.S. outbreak.
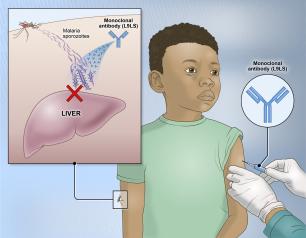
Experimental NIH Malaria Monoclonal Antibody Protective in Malian Children
April 26, 2024
One injected dose of an experimental malaria monoclonal antibody was 77% effective against malaria disease in children in Mali during the country’s six-month malaria season, according to the results of a mid-stage clinical trial. The trial assessed an investigational monoclonal antibody developed by scientists at the National Institutes of Health (NIH), and results appear in The New England Journal of Medicine.
World TB Day 2024 – Yes! We Can End TB!
March 22, 2024
In observance of World Tuberculosis Day (Sunday, March 24), NIAID joins our partners in reaffirming our commitment to ending the tuberculosis (TB) pandemic while honoring the lives lost to TB disease.
NIH Scientists Find Weak Points on Epstein-Barr Virus
March 12, 2024
Studies of interactions between two lab-generated monoclonal antibodies (mAbs) and an essential Epstein-Barr virus (EBV) protein have uncovered targets that could be exploited in designing treatments and vaccines for this extremely common virus. The research was led by Jeffrey I. Cohen, M.D., and colleagues from NIAID.

Children Surpass a Year of HIV Remission after Treatment Pause
March 6, 2024
Four children have remained free of detectable HIV for more than one year after their antiretroviral therapy (ART) was paused to see if they could achieve HIV remission, according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. The children, who acquired HIV before birth, were enrolled in a clinical trial funded by the National Institutes of Health in which an ART regimen was started within 48 hours of birth and then closely monitored for drug safety and HIV viral suppression.

Long-Acting HIV Treatment Benefits Adults with Barriers to Daily Pill Taking and Adolescents with Suppressed HIV
March 6, 2024
Long-acting, injectable antiretroviral therapy (ART) suppressed HIV replication better than oral ART in people who had previously experienced challenges taking daily oral regimens and was found safe in adolescents with HIV viral suppression, according to two studies presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Both studies were sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, in collaboration with other NIH institutes.
Semaglutide Reduces Severity of Common Liver Disease in People with HIV
March 5, 2024
A weekly injection of semaglutide was safe and reduced the amount of fat in the liver by 31% in people with HIV and metabolic dysfunction-associated steatotic liver disease (MASLD), according to a presentation today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. This is the first clinical trial of semaglutide for MASLD in people with HIV.

Vaginal Ring and Oral Pre-Exposure Prophylaxis Found Safe for HIV Prevention Throughout Pregnancy
March 5, 2024
The monthly dapivirine vaginal ring and daily oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate and emtricitabine were each found to be safe for HIV prevention among cisgender women who started using one of them in their second trimester of pregnancy, according to findings presented today at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver. Pregnant people are estimated to be three times more likely to acquire HIV through sexual intercourse than similarly aged people who are not pregnant.
Tools Underestimate Cardiovascular Event Risk in People with HIV
March 4, 2024
The elevated cardiovascular disease risk among people with HIV is even greater than predicted by a standard risk calculator in several groups, including Black people and cisgender women, according to analyses from a large international clinical trial primarily funded by the National institutes of Health and presented at the 2024 Conference on Retroviruses and Opportunistic Infections (CROI) in Denver.
New Antibodies Target “Dark Side” of Influenza Virus Protein
March 1, 2024
Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored “dark side” of the neuraminidase (NA) protein head. The antibodies target a region of the NA protein that is common among many influenza viruses, including H3N2 subtype viruses, and could be a new target for countermeasures. The research, led by scientists at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center, part of NIH, was published today in Immunity.
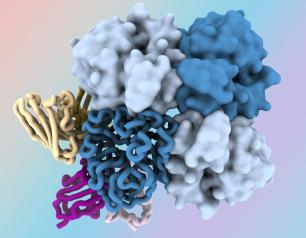
Antibody Reduces Allergic Reactions to Multiple Foods in NIH Trial
February 25, 2024
A 16-week course of a monoclonal antibody, omalizumab, increased the amount of peanut, tree nuts, egg, milk and wheat that multi-food allergic children as young as 1 year could consume without an allergic reaction in a late-stage clinical trial.

Statement: Long-Acting HIV Treatment Demonstrates Efficacy in People with Challenges Taking Daily Medicine as Prescribed
February 21, 2024
Long-acting antiretroviral therapy (ART) with cabotegravir and rilpivirine was superior in suppressing HIV replication compared to daily oral ART in people who had been unable to maintain viral suppression through an oral daily regimen, according to interim data from a randomized trial. Upon review of these findings, an independent Data and Safety Monitoring Board (DSMB) recommended halting randomization and inviting all eligible study participants to take long-acting ART.
Statement: NIH Trial Data Underpins FDA Approval of Omalizumab for Food Allergy
February 16, 2024
FDA has approved omalizumab for the reduction of allergic reactions, including anaphylaxis, that may occur with an accidental exposure to one or more foods in adults and children aged 1 year and older with food allergy. People taking omalizumab still need to avoid exposure to foods to which they are allergic.

COVID-19 Vaccination and Boosting During Pregnancy Protects Infants for Six Months
February 14, 2024
Women who receive an mRNA-based COVID-19 vaccination or booster during pregnancy can provide their infants with strong protection against symptomatic COVID-19 infection for at least six months after birth. These findings reinforce the importance of receiving both a COVID-19 vaccine and booster during pregnancy to ensure that infants are born with robust protection that lasts until they are old enough to be vaccinated.
Switching to Vegan or Ketogenic Diet Rapidly Impacts Immune System
January 30, 2024
Researchers observed rapid and distinct immune system changes in a small study of people who switched to a vegan or a ketogenic (also called keto) diet. Scientists closely monitored various biological responses of people sequentially eating vegan and keto diets for two weeks, in random order. They found that the vegan diet prompted responses linked to innate immunity—the body’s non-specific first line of defense against pathogens—while the keto diet prompted responses associated with adaptive immunity—pathogen-specific immunity built through exposures in daily life and vaccination. Metabolic changes and shifts in the participants' microbiomes—communities of bacteria living in the gut—were also observed. More research is needed to determine if these changes are beneficial or detrimental and what effect they could have on nutritional interventions for diseases such as cancer or inflammatory conditions.
Researchers Create Safer Form of Coxiella burnetii for Scientific Use
January 25, 2024
Scientists have unexpectedly discovered that the weakened form of the bacteria Coxiella burnetii (C. burnetii) not typically known to cause disease, naturally acquired an ability to do so. C. burnetii causes Q Fever in humans and its weakened forms are those used for scientific purposes. Subsequently, the scientists identified the genetic mutation responsible for the increased ability to cause disease (virulence) and created a form of the bacteria without the genetic flaw that could safely be used for research.
NIH-Developed HIV Antibodies Protect Animals in Proof-of-Concept Study
January 17, 2024
Three different HIV antibodies each independently protected monkeys from acquiring simian-HIV (SHIV) in a placebo-controlled proof-of-concept study intended to inform development of a preventive HIV vaccine for people. The antibodies—a human broadly neutralizing antibody and two antibodies isolated from previously vaccinated monkeys—target the fusion peptide, a site on an HIV surface protein that helps the virus fuse with and enter cells.