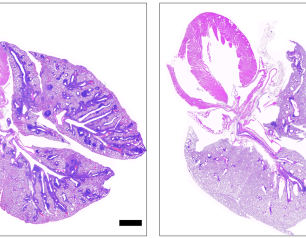
74 Results
World TB Day 2022—Invest to End TB. Save Lives
March 24, 2022
Today marks the 140th anniversary of the announcement by Dr. Robert Koch that tuberculosis (TB) is caused by the bacterium Mycobacterium tuberculosis. World TB Day is a reminder that this ancient disease remains a relentless killer. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, affirms its commitment to the 2022 World TB Day theme, Invest to End TB. Save Lives, by supporting and conducting wide-ranging research aimed at reducing the health and economic impacts of TB.
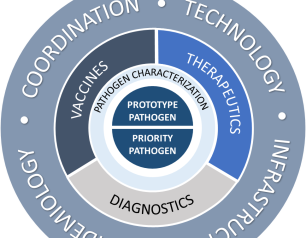
NIAID Pandemic Preparedness Plan Targets ‘Prototype’ and Priority Pathogens
February 2, 2022
The National Institute of Allergy and Infectious Diseases is focusing on preparing for a range of other viral threats that could cause a public health emergency, and according to NIAID’s new Pandemic Preparedness Plan, the institute will direct its preparedness efforts on two fronts.
Intranasal Influenza Vaccine Spurs Strong Immune Response in Phase 1 Study
February 3, 2021
An experimental influenza vaccine was safe and produced a durable immune response when tested in a Phase 1 study, NIH reports.
Tecovirimat Is Safe but Ineffective as Treatment for Clade II Mpox
March 12, 2025
Monotherapy with the antiviral drug tecovirimat was safe but ineffective as an mpox treatment in an international clinical trial.
World TB Day 2024 – Yes! We Can End TB!
March 22, 2024
In observance of World Tuberculosis Day (Sunday, March 24), NIAID joins our partners in reaffirming our commitment to ending the tuberculosis (TB) pandemic while honoring the lives lost to TB disease.
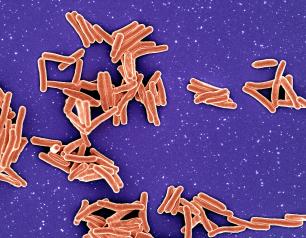
Statement—Janssen Investigational COVID-19 Vaccine—Interim Analysis
January 29, 2021
NIH reports that an investigational COVID-19 vaccine by Janssen Pharmaceuticals appears to be safe and effective at preventing COVID-19 in adults.
Hyperimmune Intravenous Immunoglobulin Does Not Improve Outcomes for Adults Hospitalized with COVID-19
January 27, 2022
A clinical trial has found that the combination of remdesivir plus a highly concentrated solution of antibodies that neutralize SARS-CoV-2, the virus that causes COVID-19, is not more effective than remdesivir alone for treating adults hospitalized with the disease.
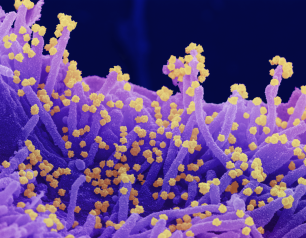
Dr. Fauci Reflects on the Perpetual Challenge of Infectious Diseases
November 28, 2022
Dr. Fauci, who since 1984 has directed the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, reflects on his career responding to infectious disease threats.

NIH Study Finds Tecovirimat Was Safe but Did Not Improve Mpox Resolution or Pain
December 10, 2024
Tecovirimat was safe but did not reduce the time to lesion resolution or reduce pain among adults with mild to moderate clade II mpox and a low risk of severe disease in an international study.
Statement by Anthony S. Fauci, M.D.
August 22, 2022
I am announcing today that I will be stepping down from the positions of Director of the National Institute of Allergy and Infectious Diseases (NIAID) and Chief of the NIAID Laboratory of Immunoregulation, as well as the position of Chief Medical Advisor to President Joe Biden. I will be leaving these positions in December of this year to pursue the next chapter of my career.
Investigational Three-Month TB Regimen Is Safe but Ineffective, NIH Study Finds
July 5, 2023
The first clinical trial of a three-month tuberculosis (TB) treatment regimen is closing enrollment because of a high rate of unfavorable outcomes with the investigational course of treatment. Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) 5362, also known as the CLO-FAST trial, sought to evaluate the safety and efficacy of a three-month clofazimine- and high-dose rifapentine-containing regimen. An interim data analysis showed that participants taking the investigational regimen experienced ongoing or recurring TB at rates above thresholds set in the study protocol.
SARS-CoV-2 Infection Weakens Immune-Cell Response to Vaccination
March 20, 2023
The magnitude and quality of a key immune cell’s response to vaccination with two doses of the Pfizer-BioNTech COVID-19 vaccine were considerably lower in people with prior SARS-CoV-2 infection compared to people without prior infection, a study has found. In addition, the level of this key immune cell that targets the SARS-CoV-2 spike protein was substantially lower in unvaccinated people with COVID-19 than in vaccinated people who had never been infected. Importantly, people who recover from SARS-CoV-2 infection and then get vaccinated are more protected than people who are unvaccinated.

Findings Suggest COVID-19 Rebound Not Caused by Impaired Immune Response
October 6, 2022
Findings from a small study of eight patients published in Clinical Infectious Diseases suggest that COVID-19 rebound is likely not caused by impaired immune responses. The study, led by scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, aimed to define the clinical course and the immunologic and virologic characteristics of COVID-19 rebound in patients who have taken nirmatrelvir/ritonavir (Paxlovid), an antiviral therapeutic developed by Pfizer, Inc.
NIAID Appoints Ted Pierson as New Vaccine Research Center Director
April 25, 2023
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has named Theodore (Ted) C. Pierson, Ph.D., as the new director of its Dale and Betty Bumpers Vaccine Research Center (VRC) in Bethesda, MD.

Statement—Large Clinical Trial Will Test Combination Monoclonal Antibody Therapy for Mild/Moderate COVID-19
January 5, 2021
A NIAID-supported clinical trial has begun to evaluate a combination investigational monoclonal antibody therapy for people with mild to moderate COVID-19.
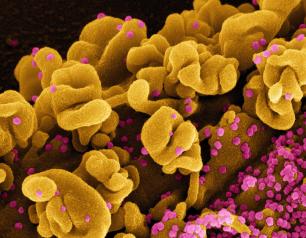
Clinical Trial of Therapeutics for Severely Ill Hospitalized COVID-19 Patients Begins
April 22, 2021
A new Phase 3 trial to test the safety and efficacy of therapeutics for COVID-19 has begun enrolling patients hospitalized with life-threatening cases of COVID-19, including those with acute respiratory failure.
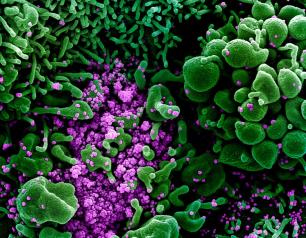
Statement—Four Potential COVID-19 Therapeutics Enter Phase 2/3 Testing in NIH ACTIV-2 Trial
February 12, 2021
Enrollment has begun to test additional investigational drugs in the NIH Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program.
Statement—Large NIH Clinical Trial Will Test Polyclonal Antibody Therapeutic for COVID-19
April 21, 2021
A Phase 2/3 trial to evaluate a new fully-human polyclonal antibody therapeutic targeted to SARS-CoV-2, called SAB-185, has begun enrolling non-hospitalized people with mild or moderate cases of COVID-19. The trial, ACTIV-2, is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. The therapeutic was developed by SAB Biotherapeutics, Inc. (Sioux Falls, South Dakota).
As Prevention Strategy for Sexually Transmitted Infections Rolls Out, Experts Highlight both Promise and Knowledge Gaps
January 6, 2025
DoxyPEP is reducing the rate of syphilis and chlamydia but has had little to no effect on gonorrhea and needs close monitoring for antibiotic resistance.
U.S. Clinical Trial Evaluating Antiviral for Monkeypox Begins
September 9, 2022
A Phase 3 clinical trial evaluating the antiviral tecovirimat, also known as TPOXX, is now enrolling adults and children with monkeypox infection in the United States. Study investigators aim to enroll more than 500 people from clinical research sites nationwide. Interested volunteers can visit the ACTG website (clinical trial A5418) for more information. The NIAID-funded Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) is leading the study, which may later expand to international sites. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at NIH is supporting several sites, including through the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT).
Monkeypox Treatment Trial Begins in the Democratic Republic of the Congo
October 12, 2022
A clinical trial to evaluate the antiviral drug tecovirimat, also known as TPOXX, in adults and children with monkeypox has begun in the Democratic Republic of the Congo (DRC). The trial will evaluate the drug’s safety and its ability to mitigate monkeypox symptoms and prevent serious outcomes, including death. NIAID and the DRC’s National Institute for Biomedical Research (INRB) are co-leading the trial as part of the government-to-government PALM partnership. Collaborating institutions include the U.S. Centers for Disease Control and Prevention (CDC), the Institute of Tropical Medicine Antwerp, the aid organization Alliance for International Medical Action (ALIMA) and the World Health Organization (WHO).
World TB Day 2023 – ‘Yes! We Can End TB!’
March 24, 2023
Each year, on March 24, the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, joins people and organizations from around the globe in marking World Tuberculosis Day. On this day, more than 140 years ago, Dr. Robert Koch announced his discovery that most human tuberculosis (TB) is caused by the bacterium Mycobacterium tuberculosis (Mtb). Although our scientific insight into this disease has grown over the past century, TB is still one of the deadliest infectious diseases on the planet. Today, NIAID joins the world in a message of hope: “Yes!
NIH Launches Trial to Study Allergic Reactions to COVID-19 mRNA Vaccine
March 9, 2022
Researchers from the National Institute of Allergy and Infectious Diseases (NIAID) are conducting a clinical trial designed to help understand rare but potentially serious systemic allergic reactions to COVID-19 mRNA vaccines.
Existing Drug Shows Promise as Treatment for Rare Genetic Disorder
May 30, 2024
A drug approved to treat certain autoimmune diseases and cancers successfully alleviated symptoms of a rare genetic syndrome called autoimmune polyendocrine syndrome type 1 (APS-1).