In 1968, an influenza pandemic swept the globe, exposing everyone to a new flu strain and wiping out all traces of the H2N2 virus subtype, which had been responsible for the 1957 pandemic and had circulated for a decade afterward. People born after 1969 have thus never been exposed to H2 subtype influenza, while those born earlier have immunological experience with it. Researchers from the NIAID Vaccine Research Center (VRC) used this difference in immunological memory to investigate immune responses to a novel influenza vaccine candidate that VRC scientists had designed and developed. The vaccine candidate was tested in a Phase 1 trial led by VRC scientists Julie Ledgerwood, D.O., and Grace Chen, M.D. It was the first time this investigational vaccine had been tested in people. Results were published earlier this year in Nature Medicine.
The trial vaccine is one of a still-experimental class of flu vaccines that could one day be used to provide long-lasting protection against multiple flu strains. Current seasonal flu vaccines work by generating protective antibodies aimed at the “head” portion of a flu glycoprotein—called hemagglutinin or HA—that protrudes like a mushroom from the virus surface. The HA head region mutates constantly, so antibodies produced following one year’s flu shot will not necessarily be able to recognize the changed HA head in subsequent seasons. Therefore, flu shots must be re-formulated yearly to match several virus strains that are predicted to predominate in the upcoming season. This time-consuming process involves propagating virus strains at large scale and must start well before the flu season begins. If the virus strains included in the vaccine end up mismatched to the circulating strains, the vaccine’s effectiveness is reduced.
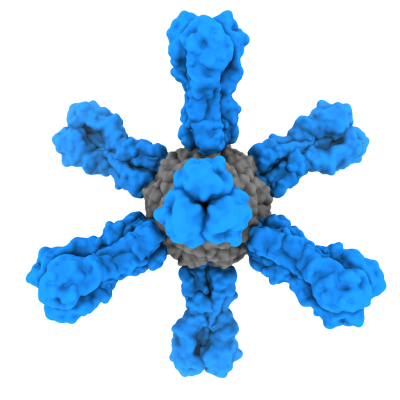
In contrast to current flu vaccines, the vaccine candidate tested by the VRC scientists did not require growing live flu virus. Instead, the vaccine was produced in bioreactors and is based on ferritin, an iron-containing protein that spontaneously self-assembles into eight-sided nanoparticles. The nanoparticles serve as scaffolding to display multiple copies of HA protein arranged in a repetitive pattern that is very stimulating to the immune system. In the case of the ferritin nanoparticle vaccine tested in the recent trial, the HA protein displayed on the scaffold was from the now-extinct H2N2 flu subtype.
In another difference from standard flu vaccines, the trial vaccine was designed to elicit antibodies not to the changeable head of HA but to its less variable “stem” portion. The stem of HA is shared across a wide range of flu subtypes and is thus an appealing target for the development of broadly protective flu vaccines. Clinical lots of the ferritin nanoparticle vaccine were manufactured by the Vaccine Clinical Materials Program at the Frederick National Laboratory.
Fifty healthy adult volunteers enrolled in the trial between October 2017 and November 2018. They were divided into those born before 1966 (and thus assumed to have been exposed to H2 flu subtype) and those born after 1969 (and never previously exposed to H2). This grouping scheme gave the investigators a chance to assess whether the H2-based vaccine would elicit different responses depending on whether the volunteer had or did not have prior exposure to H2 flu virus. This is important because any future broadly protective “universal” flu vaccine—that is, a flu vaccine that provides immunity to a broad range of flu viruses—must be able to overcome pre-existing immunological memory resulting from the vaccinated person’s prior exposure to the HA head region of viruses that circulated in past flu seasons.
Volunteers received two injections spaced 16 weeks apart. The vaccine was safe and well tolerated. Neutralizing antibody production was measured before the first injection, four weeks after the first injection, and two weeks after the second injection. As expected, none of the younger volunteers had antibodies to H2N2 flu subtype prior to receiving vaccine, while about half of the older volunteers did. Neutralizing antibodies were detected in all trial participants after the first vaccine dose, regardless of previous H2 exposure. Further analysis indicated that broadly neutralizing antibodies aimed at the HA stem were produced after a single dose of vaccine in all the H2-naïve participants. The increase in vaccine-induced, stem-directed broadly neutralizing antibodies was considerably smaller among the H2-exposed participants but was detectable. The neutralizing antibody response was durable, the researchers found, persisting for six months after the second injection.
“Owing to the breadth of response induced, these results indicate a potential use for this ferritin nanoparticle-based antigen display platform in pandemic vaccine preparedness and for universal influenza vaccine development,” the researchers concluded.
In a related study also published in Nature Medicine, VRC researchers led by Adrian McDermott, Ph.D., performed further analyses of samples from this trial and another VRC-led clinical trial that immunized participants with inactivated influenza H5N1 vaccine. The aim was to study stem-specific B cell responses to influenza HAs. HAs can be divided into groups based on their amino acid sequence, with genetically similar HAs placed in the same group. Current seasonal quadrivalent influenza vaccines contain two Influenza A viruses—one (H1) from Group 1 HA and one (H3) from Group 2 HA—and two Influenza B viruses. The H5N1 and H2N2 influenza viruses have Group 1 HAs with pandemic potential. H5N1 has never circulated widely among humans, while H2N2 circulated globally only between 1957 and 1966.
In adults, the HA stem-specific B cell response to flu vaccination is largely through expansion and activation of pre-existing memory B cells formed following previous flu infections or vaccinations. In H2-naïve individuals, who had been exposed only to the Group 1 subtype H1 prior to their participation in the trials, H2 vaccination led to a more cross-reactive and broadly neutralizing B cell response than H5 vaccination. The researchers determined that a single amino acid, unique to the H2 stem, caused the expansion of more cross-reactive HA stem-binding memory B cells. In older individuals, where these pre-existing memory B cells were formed during H2 flu exposure a half century ago, the response to both H2 and H5 vaccination was broadly neutralizing. The study found that whether trial participants received the H2 vaccine candidates or had been exposed to H2 when it was still circulating more than 50 years ago, the immune response targeted a region in the HA stem that is unique to H2. The memory B cells and antibodies targeting this H2 stem region were capable of recognizing and neutralizing influenza subtypes across Group 1, suggesting that it which may hold promise for the development of future universal flu vaccines.
References: KV Houser, et al. Safety and immunogenicity of a ferritin nanoparticle H2 influenza vaccine in healthy adults: a phase 1 trial. Nature Medicine DOI: https://www.nature.com/articles/s41591-021-01660-8 (2022).
SF Andrews, et al. A single residue in influenza virus H2 hemagglutinin enhances the breadth of the B cell response elicited by H2 vaccination. Nature Medicine DOI: https://www.nature.com/articles/s41591-021-01636-8 (2022).