May 18, 2023
Hugh Auchincloss, M.D., Acting Director, National Institute of Allergy and Infectious Diseases
Bill G. Kapogiannis, M.D., Acting NIH Associate Director for AIDS Research and Acting Director of the NIH Office of AIDS Research (OAR)
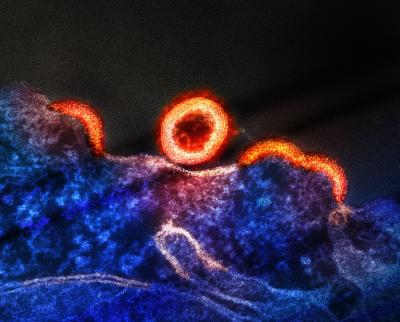
A micrograph of HIV-1 virus particles from an HIV-infected H9 T-cell, shown in blue and purple. Budding virus particles that have not yet separated from the cell appear as semi-circles. A separated, spherical immature particle is seen at center of the image. Image captured by a colorized transmission electron microscope at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
Today marks the 26th observance of HIV Vaccine Awareness Day. The National Institutes of Health applauds the efforts of the collaborative global community of scientists, advocates, study participants, study staff, and funders enabling unprecedented levels of innovation and adaptation in the pursuit of a highly effective HIV vaccine. HIV continues to pose formidable challenges to vaccine development due to its ability to mutate rapidly and hide in “reservoirs” that the immune system cannot reach. These unique attributes have compelled scientists to apply cutting-edge tools of immunology and vaccinology to HIV, while maintaining a nimble research network capable of reacting quickly as new discoveries are made.
HIV vaccine science is making headway on promising concepts that address the diverse HIV strains circulating worldwide, including harnessing broadly neutralizing antibodies (bNAbs) that can block most strains of the virus from entering immune cells. bNAbs were initially identified in people living with HIV who donated blood samples for research. The NIAID Vaccine Research Center (VRC)—founded to accelerate HIV vaccine research on this day in 1997—isolated and manufactured the bNAb VRC01. VRC01 was evaluated in the multinational Antibody-Mediated Prevention (AMP) studies as an infusion administered every eight weeks and was found to prevent acquisition of 75% of the HIV strains that were sensitive to that antibody. Insights from that research contributed to further development of long-acting antibody-based HIV prevention strategies.
Through the combined support of NIAID and the Bill & Melinda Gates Foundation, investigators at the Scripps Research Institute have developed a vaccine that uses germline targeting, a technique that closely guides naïve (new) B cells to develop into mature B cells that can produce bNAbs that work against a wide variety of HIV strains. The International AIDS Vaccine Initiative recently conducted a proof-of-concept study using the VRC01 germline-targeting protein immunogen, as well as laboratory technology developed by the VRC, and found the vaccine generated B cells capable of maturing to produce VRC01-like bNAbs in 97% of vaccinated participants. NIAID and its partners are now testing more advanced mRNA vaccine designs to determine whether mRNA can instruct the body to manufacture proteins that would teach the body to make bNAb-producing B cells in a similar fashion. Those mRNA studies will report results in the coming year. Scientists continue working to identify next-generation immunogens with potential to stimulate the body to produce bNAbs that work against an even broader variety of HIV strains. The VRC has identified antigens that include the fusion peptide—a site on HIV that helps the virus fuse with a cell to infect it—as a vaccine target for further development.
Other new HIV vaccine designs are entering early-stage clinical studies in the coming year, including a Phase 1 study to test the safety of a novel T-cell vaccine using an attenuated (weakened) cytomegalovirus vector and its ability to generate an HIV-specific immune response in healthy volunteers. An attenuated viral vector does not cause disease. Instead, it serves as a delivery system for vaccine contents. The approaches under study look to combine two different components of the immune system, bNAbs and T cells, to optimize chances of preventing HIV acquisition.
By the time a vaccine candidate reaches clinical studies—studies in people—the technology has already been explored through extensive basic and preclinical animal research. NIAID maintains a robust pipeline of promising vaccine concepts that build on the outcomes of clinical studies, ensuring vaccine development rapidly and continuously builds on past work. In this regard, NIAID has a targeted portfolio of extramural and intramural HIV vaccine research, including investments in germline targeting and lineage-based design—strategies to activate rare bNAb-producing B-cell lineages—through the $49 million Consortium for HIV/AIDS Vaccine Development awarded in 2019, and the $34 million Centers for HIV Structural Biology awarded in 2022. In the latter, scientists are illuminating detailed three-dimensional structures of HIV proteins and nucleic acids—genetic components— to better understand how different HIV genetic and immune system components interact and discover new approaches for disrupting those interactions, potentially leading to new targets for HIV vaccines.
In all these activities, NIH ensures that the needs of diverse communities are considered and emphasizes that community voices are essential to continued progress. NIH welcomes input from community members on HIV research priorities with ongoing listening sessions hosted by the NIH Office of AIDS Research. Efforts are underway to ensure that vaccine research studies engage underserved and marginalized population groups, including transgender people, to remedy low representation in prior studies. This work has led to improvements in the recent multinational MOSAICO study, in which transgender women were 10% of trial participants in Peru. In 2021, the NIAID-funded HIV Vaccine Trials Network launched the Faith Initiative, engaging clergy and community across the United States to discuss vaccine safety, sexual health, and stigma within the context of participation in a vaccine clinical trial.
As HIV vaccine research progresses, insights from this work also are enabling a fast response to other emerging health threats—ultimately supporting the health of people with HIV and broader U.S. and global communities. Community outreach through HIV research networks established the infrastructure for including diverse populations in vaccine trials for COVID-19. HIV vaccine research also has helped identify innovative technologies for the delivery of other vaccines.
For people living with or vulnerable to HIV exposure, breakthroughs in biomedical innovations continue to expand available options for treatment and prevention of HIV. These lifesaving interventions have contributed to reductions in HIV incidence and AIDS-related deaths, but all require pill-taking or routine injections to prevent or clear HIV infection. Therefore, an effective, acceptable, safe and long-lasting HIV vaccine remains crucial for ending the HIV pandemic worldwide.
As we reflect on our progress today, and more than 40 years of HIV research and development, NIH reaffirms its commitment to generating scientific solutions that could end HIV.
Contact
Submit a Media Request
Contact the NIAID News & Science Writing Branch.

