Antiviral medicines are an important tool in both controlling influenza by treating the patient’s infection and helping to prevent severe illness that can result from flu, including bacterial pneumonia. When taken promptly, antiviral drugs can reduce the severity of the flu’s worst symptoms, and can shorten the length of the illness by an average of one day. Taking antiviral drugs early is especially important for people who are at high risk for flu complications, such as the elderly or people with compromised immune systems.
Currently, there are three antiviral drugs recommended for treating the flu: oseltamivir (Tamiflu®), zanamivir (Relenza®), and peramivir (Rapivab®). These drugs work by interrupting the function of neuraminidase on the virus surface and preventing the release of viral particles from infected host cells. These drugs can treat cases of both influenza A and B and have the biggest impact when taken within 48 hours of the onset of flu symptoms, so rapid diagnosis and treatment are important.
Antimicrobial Resistance is a Problem
Two other drugs, rimantadine (Flumadine®) and amantadine (Symmetrel®), were used to treat flu infection in the past. They worked by disrupting ion channels in the wall of the virus, preventing the virus from replicating during the initial stages of infection, soon after its been taken inside a human cell. However, rimantadine and amantadine are only effective in treating Influenza A, and several strains of flu have already developed resistance to them. Thus, the two medicines are no longer recommended in the United States for treating the flu.
NIAID Flu Treatment Research
Because the influenza virus can develop resistance to antiviral drugs, NIAID is working to find new and better treatments to fight the flu. These efforts include supporting the development and testing of the next generation of antiviral drugs. For example, NIAID supported the initial Phase 1 clinical studies of peramivir, which was approved in 2014 by the Food and Drug Administration to treat flu infection in adults. This is the first neuraminidase inhibitor available in intravenous formulation, which is especially needed to deliver the effective drug dose to hospitalized patients who cannot take an oral or inhaled drug. NIAID has advanced the development and testing of additional next-generation neuraminidase inhibitors, as well as flu RNA polymerase inhibitors and monoclonal antibodies targeting the flu surface protein hemagglutinin.
Additionally, NIAID has provided support for the following:
- DAS 181-novel host-based antiviral agent: NIAID works closely with the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority (BARDA) to move candidate antivirals from early to advanced development. NIAID supported the preclinical and clinical development of DAS 181, a new class of antiviral therapeutic candidate that inhibits the influenza virus from attaching to host cells. BARDA supported a Phase 2b clinical trial of DAS 181.
- Broadly reactive monoclonal antibodies (MAbs) CR6261 and CR8020: These candidate immunotherapeutics target the stem region of influenza HA glycoprotein were discovered and developed by Crucell and supported by NIAID contract funding. CR621 targets the Flu A Group 1 HA stem, which includes flu types H1, H2, H5 and H9. CR8020 targets the Flu A group 2 HA stem, which includes H3, H7 and H10 flu subtypes. Phase 1 studies were completed in 2013.
Three NIAID clinical trials are currently exploring the effectiveness of novel flu therapeutics in high-risk populations, including human plasma containing high levels of anti-flu antibodies, concentrated human immunoglobulin with high levels of anti-flu antibodies, and a cocktail of the three licensed flu antiviral medicines.
Scientific Advances
Bovine H5N1 Influenza from Infected Worker Transmissible and Lethal in Animal Models
October 28, 2024Bovine H5N1 influenza virus taken from eye of infected worker transmissible and lethal in animal models.
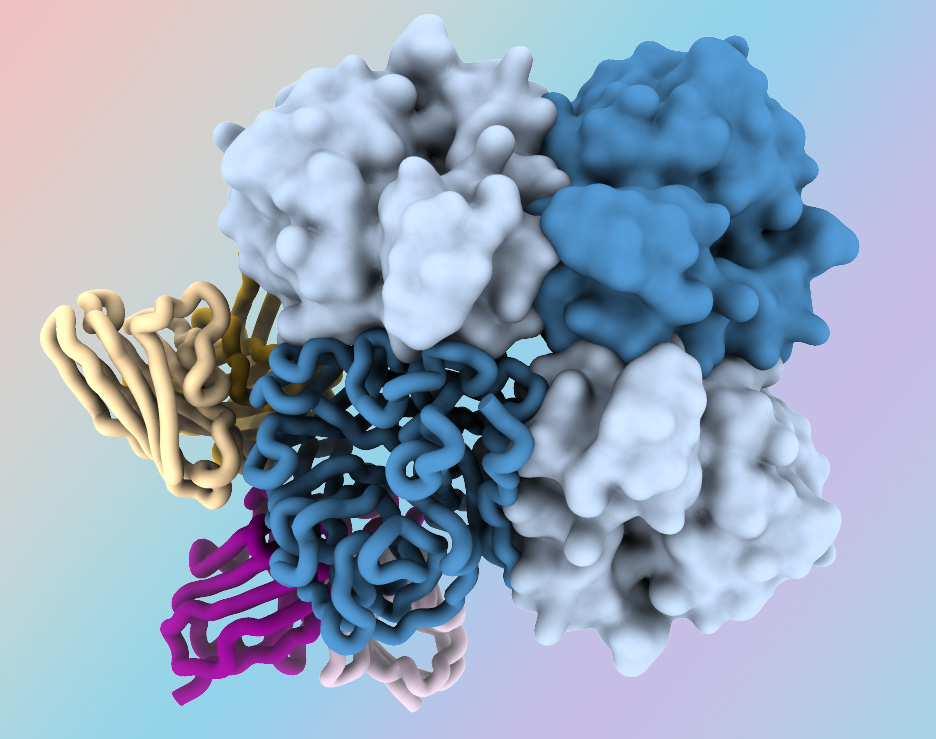
New Antibodies Target “Dark Side” of Influenza Virus Protein
March 1, 2024Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored “dark side” of the neuraminidase (NA) protein head. The antibodies target a region of the NA protein that is common among many influenza viruses, including H3N2 subtype viruses, and could be a new target for…