NIAID conducts and supports research to better understand, treat, and ultimately prevent infectious and immune-mediated diseases. For more than 60 years, NIAID research has led to new therapies, vaccines, diagnostic tests, and other technologies that have improved the health of millions of people in the United States and abroad. This page highlights notable scientific advances made by NIAID laboratories and NIAID-funded researchers.
NIAID Research Journeys
Explore the stories behind how NIAID-funded basic and clinical research paved the way for the development of important therapeutics and vaccines.
Decades in the Making: mRNA COVID-19 Vaccines
Decades of NIH-supported laboratory research laid the groundwork for the rapid development of mRNA vaccines in the first 100 days of the COVID-19 pandemic. Today, two U.S. Food and Drug Administration (FDA)-approved mRNA vaccines have saved millions of lives.

First FDA-Approved Drug for Delaying T1D Onset
Decades of NIH investment in basic research and clinical trials led to the approval of teplizumab, the first FDA-approved drug for delaying the onset of Type 1 diabetes in people at risk. This delay reduces the potential for severe long-term complications, thereby improving quality of life.
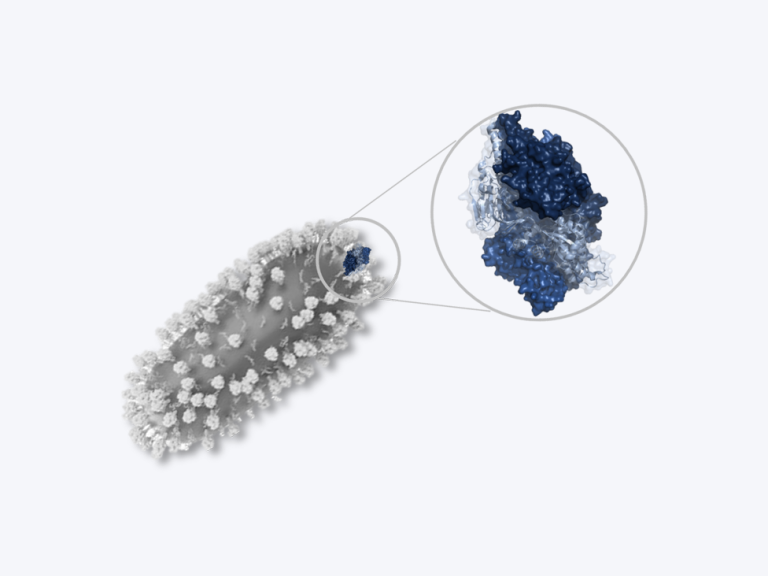
Safe and Effective RSV Protein Vaccines
NIAID-funded basic and clinical studies helped establish the fundamental knowledge necessary for the private sector to develop protein vaccines. These vaccines are safe and effective at preventing severe RSV in some target populations.


