Vaccine Production Program

Major Areas of Research
- Rapid advancement of vaccines and biologics to meet the ever-evolving needs of the Vaccine Research Center clinical pipeline, including:
- Design and generation of cell lines for high quality expression
- Exploration of bioprocess parameters for robust and scalable expression
- Design of complex purification methods for scalable production
- Biophysical characterization and stabilization for clinical use
- Quality attribute assessment and structural characterization
- Application of data interpretation of large datasets to enhance understanding of vaccine and biologic development
View all research conducted at the Vaccine Research Center (VRC)
Program Description
The Vaccine Production Program (VPP) applies cutting-edge science and engineering approaches to plasmid design, cell expression, purification, formulation, and analytics. The multidisciplinary nature of the work requires expertise in a number of scientific fields, including molecular and cellular biology, biochemistry, biophysics, biomedical and chemical engineering, and traditional pharmaceutics. The broad portfolio of knowledge, coupled with a strong culture of collaboration and innovation, makes the VPP one of the most versatile product development organizations in infectious disease research. Notable achievements include the development of novel quadrivalent and hexavalent mosaic nanoparticle vaccines to fight influenza, as well as breakthroughs in production and stabilization of trimeric protein vaccine candidates for human immunodeficiency virus (HIV) and respiratory syncytial virus (RSV).
The Vaccine Production Program portfolio includes a diverse array of both traditional and novel vaccine technologies and biologics, including:
- DNA
- Protein Subunits
- Peptide-Carrier Protein Conjugates
- Enveloped and Non-Enveloped Virus-Like Particles
- Protein Nanoparticles
- Mosaic Multi-Component Nanoparticles
- Monoclonal Antibodies
- Multi-specific Antibodies
- mRNA-Lipid Nanoparticles
- Adjuvants

Structure of Influenza Mosaic Multi-Component Nanoparticle. Left: 3D model of hexa-valent nanoparticle. Center: 2D Cryo-EM image of purified nanoparticle. Right: 3D reconstruction of 2D Cryo-EM data.
VPP Research and Technology
The Vaccine Production Program (VPP) is a science-driven organization that exists as a unique bridge between government and the biopharmaceutical industry. Research at the VPP combines the rigor of academic research with cutting-edge development. Specialized teams of scientists and engineers work in close collaboration with each other, other labs and programs within the VRC and the NIH, as well as with academic and industry partners.
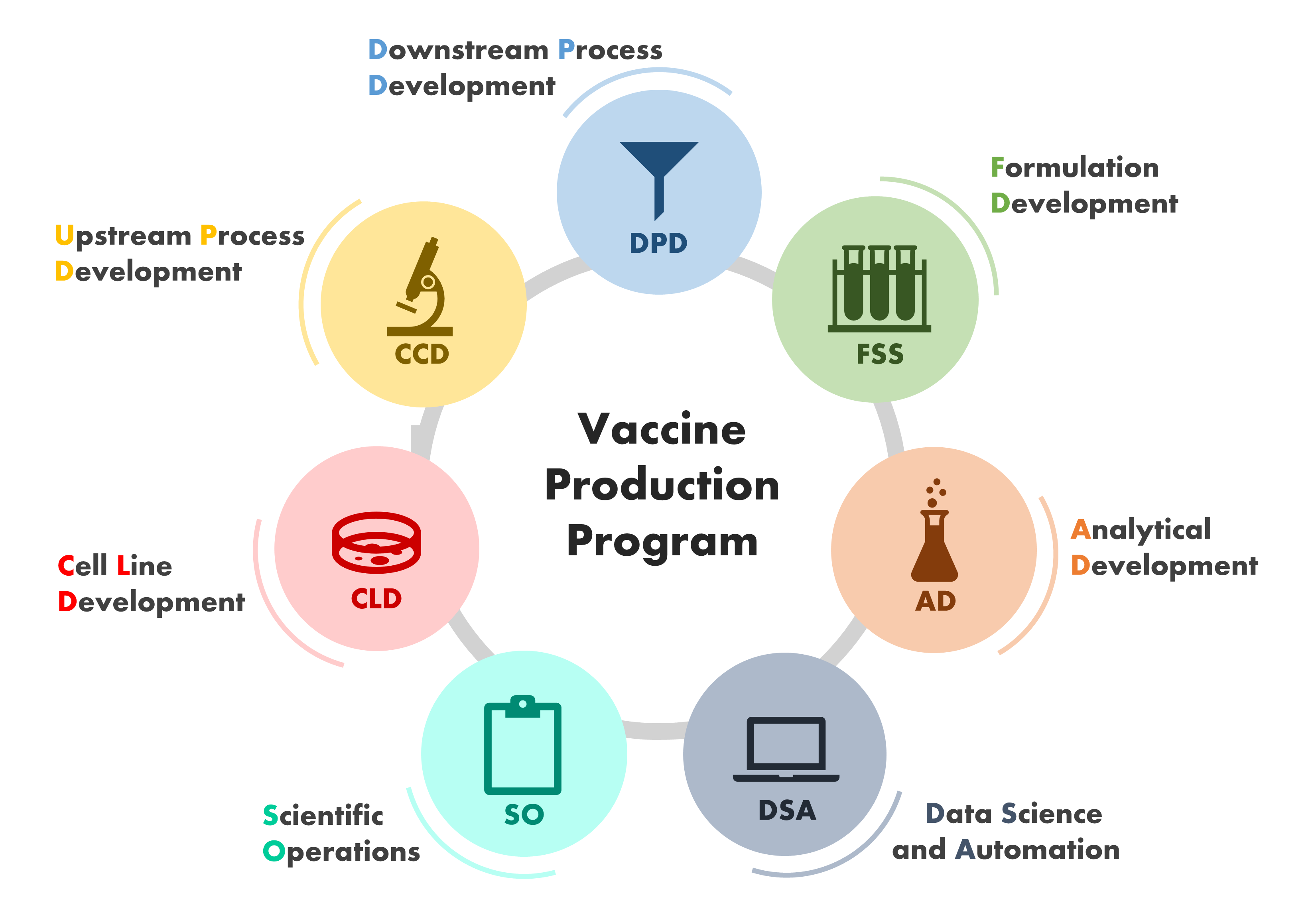
The specialized teams of scientists and engineers that work in close collaboration at the VPP are depicted in this image
Cell Line Development Group
The Cell Line Development group is responsible for generating cell lines that produce high and consistent levels of a target vaccine or therapeutic protein. This includes expression vector construction, transfection of the production host cell line, and generation of stable cell pools, followed by single-cell isolation, scale-up, and evaluation for most vaccine products. The final cell bank is ultimately used to support current good manufacturing practice (cGMP) manufacturing of the clinical trial material.
Left: High throughput cell growth monitoring. Center: Cell suspension. Right: Automated platform for cell culture, plating, and sampling
Upstream Process Development Group
Research in Upstream Process Development centers on the identification and optimization of process parameters to support and control the growth of a mammalian or microbial cell line in a production bioreactor. The resulting process must ensure a robust and scalable method to deliver high-yielding expression of the recombinant vaccine or other protein of interest. Development begins with automated microbioreactors (15 to 250 mL) and progressively increases from 3 L bench-scale bioreactors up to a pilot scale of 50 L. The finalized processes are then transferred externally to enable larger scale manufacturing of clinical material under cGMP regulations.

Left: Automated micro-bioreactors evaluating growth conditions. Right: Cell culture production at 50 L scale.
Downstream Process Development Group
Downstream Process Development employs diverse purification techniques to produce high-quality recombinant products from mammalian or microbial culture harvests. The unit operations include bulk filtration (removal of cells, cell debris, or other particulate matter), specialized chromatography steps (to separate soluble impurities like host cell proteins and DNA), and specialized filtration and/or buffer exchange steps. Ultimately, the process is transferred externally for cGMP execution at scales supporting up to 2000 L cell culture harvests. At this manufacturing scale, a high-yielding process is capable of delivering up to kilogram yields of clinical product.

Left: Small scale purification system. Center: High throughput parallel ultrafiltration system. Right: Mid-scale gel chromatography column.
Analytical Development Group
The goal of analytical method development is to design and qualify analytical methods in areas such as specific binding activity, physicochemical attributes, impurity levels, etc. These methods are applied to the characterization and evaluation of product quality, defined by its biological activity (potency), identity, purity, and stability. The Analytical Development group is also responsible for transfer of these methods to the Quality Control laboratory of a cGMP manufacturing site.

Left: High throughput chemiluminescence platform. Center: Ultra Performance Liquid Chromatography systems. Right: Automated Western Blot system.
Formulation Development Group
The Formulation Development group is responsible for developing formulations that ensure the quality of the vaccine candidates through cGMP manufacturing and clinical trials. Factors such as physical stress, temperature, light, and atmospheric oxygen can affect the physical and chemical stability of the vaccine. The Formulation Development group utilizes high-throughput methods focusing on molecular conformational, colloidal, and thermodynamic properties to identify optimal stabilizing solution conditions and additives.

Left: High throughput Background Membrane Imaging particle counter. Center: Loading a micro-volume Fluorescence cuvette. Right: Automated ultrafiltration liquid handler.
Scientific Operations Group
Scientific Operations facilitates the scientific mission of the VPP through the management of essential support systems that ensure efficient day-to-day operations. These systems include procurement, management of critical reagents and materials, safety, document retention, shipping/receiving, and coordination with NIH/NIAID groups and collaborators, including clinical sites worldwide.

Left: Shipping biologics for transport. Center: Aliquoting critical reagents. Right: Transferring cell banks to liquid nitrogen storage
Selected Publications
Yang Y, Bastani N, Lagler SK, Harris D, Nagy A, Chen P, Patel A, Li Y, Gowetski DB, Lei QP. Development and application of an analytical approach to assess an antibody's potential for disulfide reduction. Biotechnol Prog. 2022 Mar;38(2):e3229.
Loomis RJ, DiPiazza AT, Falcone S, Ruckwardt TJ, Morabito KM, Abiona OM, Chang LA, Caringal RT, Presnyak V, Narayanan E, Tsybovsky Y, Nair D, Hutchinson GB, Stewart-Jones GBE, Kueltzo LA, Himansu S, Mascola JR, Carfi A, Graham BS. Chimeric Fusion (F) and Attachment (G) Glycoprotein Antigen Delivery by mRNA as a Candidate Nipah Vaccinev. Front Immunol. 2021 Dec 8;12:772864.
Cibelli NL, Arias GF, Figur ML, Khayat SS, Leach KM, Loukinov I, Gulla KC, Gowetski DB. Advances in Purification of SARS-CoV-2 Spike Ectodomain Protein Using High-Throughput Screening and Non-Affinity Methods. Res Sq [Preprint]. 2021 Aug 20:rs.3.rs-778537.
Gaudinski MR, Berkowitz NM, Idris AH, Coates EE, Holman LA, Mendoza F, Gordon IJ, Plummer SH, Trofymenko O, Hu Z, Campos Chagas A, O'Connell S, Basappa M, Douek N, Narpala SR, Barry CR, Widge AT, Hicks R, Awan SF, Wu RL, Hickman S, Wycuff D, Stein JA, Case C, Evans BP, Carlton K, Gall JG, Vazquez S, Flach B, Chen GL, Francica JR, Flynn BJ, Kisalu NK, Capparelli EV, McDermott A, Mascola JR, Ledgerwood JE, Seder RA; VRC 612 Study Team. A Monoclonal Antibody for Malaria Prevention. N Engl J Med. 2021 Aug 26;385(9):803-814.
Gulla K, Cibelli N, Cooper JW, Fuller HC, Schneiderman Z, Witter S, Zhang Y, Changela A, Geng H, Hatcher C, Narpala S, Tsybovsky Y, Zhang B, Vrc Production Program, McDermott AB, Kwong PD, Gowetski DB. A non-affinity purification process for GMP production of prefusion-closed HIV-1 envelope trimers from clades A and C for clinical evaluation. Vaccine. 2021 Jun 8;39(25):3379-3387.
Chen P, Chen M, Menon A, Hussain AI, Carey E, Lee C, Horwitz J, O'Connell S, Cooper JW, Schwartz R, Gowetski DB. Development of a High Yielding Bioprocess for a Pre-fusion RSV Subunit Vaccine. J Biotechnol. 2021 Jan 10;325:261-270.

