The NIAID Genomic Centers for Infectious Diseases (GCID) provide insights into the biology of microbes, their role in pathogenesis, and their interactions with the host, including the microbiome, by supporting a diverse set of genomic capabilities, such as high-throughput sequencing and related genomic technologies.
Main Areas of Focus
- Develop and use high-throughput sequencing and related genomic technologies such as transcriptomics and metagenomics of microorganisms, vectors, hosts and host microbiomes to address knowledge gaps
- Develop comparative genomic analysis tools for genotyping and variant identification as well as phylogeny inference
- Implement and improve gene expression techniques for pathogenic microorganisms to understand pathogen and gene functions
- Provide bioinformatics software tools, analyses, methods and protocols to the scientific community
- Provide sequencing, technology, and bioinformatic infrastructure to address and respond to pathogen outbreaks
Contact Information
The Center for Advancing Genomic, Transcriptomic and Functional Approaches to Combat Globally Important and Emerging Pathogens
Principal Investigator/Director: Daniel Neafsey, Ph.D.
Institute: Broad Institute, Boston, Massachusetts
The Center for Integrated Genomics of Mucosal Infections
Principal Investigator/Director: Joseph Petrosino, Ph.D.
Institute: Baylor College of Medicine, Houston, Texas
The Michigan Infectious Disease Genomics (MIDGE) Center
Principal Investigator/Director: Adam Lauring, Ph.D.
Institute: The University of Michigan, Ann Arbor, Michigan
The University of Maryland Genomics Center (ends 2025)
Principal Investigator/Director: David Rasko, Ph.D.
Institute: The University of Maryland, Baltimore, Maryland
Centers of Excellence for Translational Research (CETR)
In 2014, NIAID established the Centers of Excellence for Translational Research (CETR) program. Supported translational activities will range from very early discovery-based efforts to late-stage preclinical development.
The CETR program builds on work of previous NIAID-supported research and development programs, including the Regional Centers of Excellence for Biodefense and Emerging Infectious Diseases Research (RCE) program, and is intended to complement and enhance ongoing translational and product development activities.
Main Areas of Focus
- To advance discovery, preclinical development, production, licensure and/or use of new or improved medical countermeasures (therapeutics, immunotherapeutics, vaccines, vaccine technologies, and medical diagnostics) or related technologies for emerging and re-emerging infectious diseases
Contact Information
Each multi-project Center is organized around specific themes that address development of a targeted medical countermeasure or technology, and related regulatory barriers.
Bioinformatics Resource Centers for Infectious Diseases Program
The Bioinformatics Resource Centers (BRCs) for Infectious Diseases program was initiated in 2004 with the main objective of providing public access to computational platforms and analysis tools that enable collecting, archiving, updating, and integrating a variety of genomics and related research data relevant to infectious diseases and pathogens and their interaction with hosts.
Main Areas of Focus
- To provide integrated knowledgebases, computational infrastructure, and technical expertise to make bioinformatics data (genomics, transcriptomics, metabolomics, proteomics, metagenomics, surveillance, etc.) and services available. The goal is to accelerate basic and applied infectious diseases bioinformatics research that is accessible to the broader infectious diseases research community through web and command-line interfaces
- To advance bioinformatics technologies by developing new software tools and incorporating production-level implementation of community-driven emerging tools, software, or technologies
- To offer bioinformatics trainings, educational materials, and other community outreach activities in the US and globally
- To provide bioinformatics resources and analytics in response to emerging needs, outbreaks, and public health emergencies
Awards
Awardee: University of Chicago
Principal Investigator/Director: Rick Stevens, Ph.D.
Award number: U24AI183849
Website: Bacterial and Viral Bioinformatics Resource Center (BV-BRC)
Biological Domains: Bacteria, Archaea, Viruses, and Hosts of Human Infectious Diseases
Awardee: Pennsylvania State University
Principal Investigator/Director: Anton Nekrutenko, Ph.D.
Award number: U24AI183870
Website: The Bioinformatics Resource Analytics Center (BRC.analytics)
Biological Domains: Eukaryotic Pathogens, Bacteria, Viruses, Vectors, and Hosts of Human Infectious Diseases
Awardee: Swiss Institute of Bioinformatics
Principal Investigator/Director: Aitana Neves, Ph.D.
Award number: U24AI183840
Website: The Pathogen Data Network (PDN)
Biological Domains: Eukaryotic Pathogens, Bacteria, Viruses, Vectors, and Hosts of Human Infectious Diseases
Contact Information
- Dr. Wiriya Rutvisuttinunt, DMID Office of Genomics and Advanced Technologies
The BRCs specialize on the following groups of pathogens, relevant vectors, and hosts:
- Pathogenic bacteria and closely related species
- Human viral families and those of pandemic potential
- Eukaryotic human pathogen species including fungi, parasites, and metazoan pathogens
- Relevant vectors and hosts
Successful NCBI-NIAID Codeathon Collaborating on Antimicrobial Resistance Data Analysis Projects
The National Center for Biotechnology Information (NCBI) and NIAID hosted a successful virtual event, "Resistance is Futile: A codeathon to combat antimicrobial resistance," on September 23-27, 2004. The codeathon brought together experts in the field of antimicrobial resistance and those with bioinformatic, data science skills from around the world.

NIAID Funds Cutting-Edge Genomic and Bioinformatic Programs
NIAID has announced six awards totaling approximately $19.1 million per year to continue the Genomic Centers for Infectious Diseases (GCIDs) and Bioinformatics Resource Centers (BRCs) for Infectious Diseases, both important data science networks offering critical resources for the scientific community. The GCIDs and BRCs provide public access to high-quality genomic data and data analytics technologies, tools, and training to facilitate discoveries by researchers studying viruses, bacteria, fungi, parasites, other eukaryotic pathogens, and vectors.
International Centers of Excellence for Malaria Research (ICEMR)
Main Areas of Focus
Each Center aims to:
- Design and conduct multidisciplinary research on the epidemiology, transmission, and pathogenesis of malaria in its specific region
- Design and conduct special projects to capitalize on new opportunities and emerging public health needs
- Build clinical research capacity and improve malaria control and prevention
Recognizing the urgent need for a multidisciplinary approach to malaria research, in 2010, NIAID established the International Centers of Excellence for Malaria Research (ICEMRs) to address the complex interactions at the molecular, cellular, and field levels in malaria-endemic regions.
The ICEMR program created a global network of independent research centers in malaria-endemic settings to provide knowledge, tools, and evidence-based strategies to support researchers working in a variety of settings, especially within governments and healthcare institutions.
The ICEMRS are a global network of independent research centers in malaria-endemic settings, established by NIAID to provide the knowledge, tools, and evidence-based strategies crucial to understanding, controlling, and, ultimately, preventing malaria.
Antibacterial Resistance Leadership Group (ARLG)
Read more about this network: Antibacterial Resistance Leadership Group (ARLG)
Main Areas of Focus
The renewed ARLG program is focused on three priorities:
- Infections caused by Gram-negative bacteria, such as Escherichia coli, Klebsiella pneumoniae, and other carbapenem-resistant Enterobacteriaceae (CRE)
- Infections caused by Gram-positive bacteria, such as methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococci
- Diagnostic tests to optimize use of antibiotics
For more information on the ARLG's accomplishments and scientific direction, please see The Antibacterial Resistance Leadership Group (ARLG): Innovation and Evolution (Clin. Infec. Dis., Volume 77, Supplement, 2023 Oct 15).
Mentoring and Guidance
In addition to developing, implementing, and managing an extensive clinical program, the ARLG is committed to mentoring the next generation of clinical scientists in the field of AR. The ARLG offers three opportunities to meet the needs of trainees at different career stages: the ARLG Fellowship, Early Stage Investigator Seed Grants, and the Trialists in Training Program. For more information, please visit https://arlg.org/. Experts from the ARLG are also available to consult with industry and provide advice, guidance, and protocol design for drug, device, or diagnostic development.
Oversight
The ARLG is led by two principal investigators, Dr. Vance Fowler, Duke University, and Dr. Henry Chambers, University of California, San Francisco. Work is supported by ARLG’s expert committees and the Duke Clinical Research Institute. NIAID/Division of Microbiology and Infectious Diseases (DMID) and an External Advisory Board provide oversight and direction for the ARLG.
The ARLG has three separate component centers:
- Scientific Leadership Center, directed by Drs. Vance Fowler and Chip Chambers
- Clinical Operations Center, directed by Dr. Heather Cross, Duke Clinical Research Institute
- Statistics and Data Management Center, directed by Dr. Scott Evans, Harvard University
- Laboratory Center, led by Dr. Robin Patel, Mayo Clinic
NIAID conducts and supports research on antimicrobial resistance. Because most bacteria, viruses, and other microbes multiply rapidly, they can quickly evolve and develop resistance to antimicrobial drugs.
- NIH-Supported Clinical Trial of Phage Therapy for Cystic Fibrosis Begins – October 4, 2022
- Trial of Existing Antibiotic for Treating Staphylococcus aureus Bacteremia Begins – April 27, 2021
- Childhood Pneumonia Study Shows Short-Course Antibiotics Superior to Standard of Care – October 23, 2020
- NIH Renews Funding for the Antibacterial Resistance Leadership Group – December 13, 2019
- Study Supports Expanded Testing for Gonorrhea and Chlamydia – July 1, 2019
In 2013, NIAID launched the Antibacterial Resistance Leadership Group (ARLG), a major clinical effort to address antibacterial resistance (AR). Since its inception, the program has established collaborations in 19 countries and conducted over 40 clinical research studies involving more than 20,000 volunteers. Studies conducted by the ARLG include clinical testing of new drugs to treat multidrug-resistant Gram-negative bacteria, evaluating diagnostic devices in clinical settings, and optimizing treatment regimens to reduce the emergence of resistance. In 2019, NIAID renewed the program’s funding, providing up to $102.5 million over 7 years.
Highlights
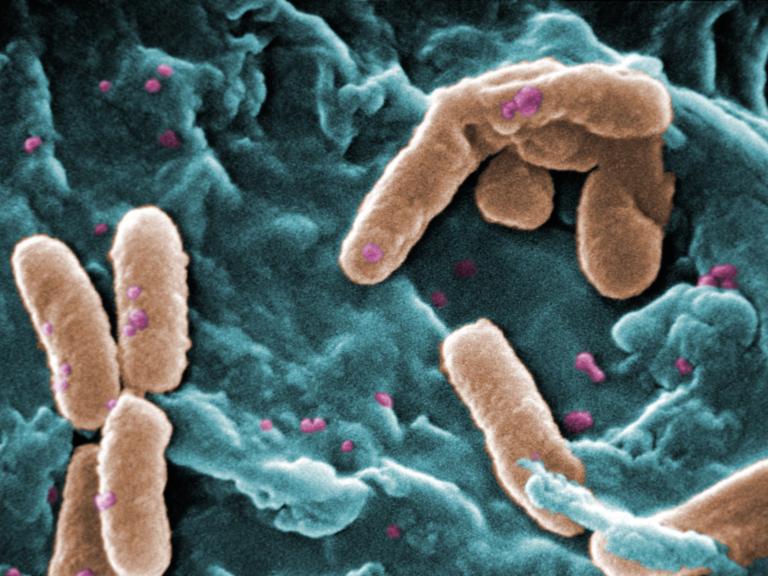
NIH-Supported Clinical Trial of Phage Therapy for Cystic Fibrosis Begins
Enrollment has begun in an early-stage clinical trial evaluating bacteriophage therapy in adults with cystic fibrosis (CF) who carry Pseudomonas aeruginosa (P. aeruginosa) in their lungs.
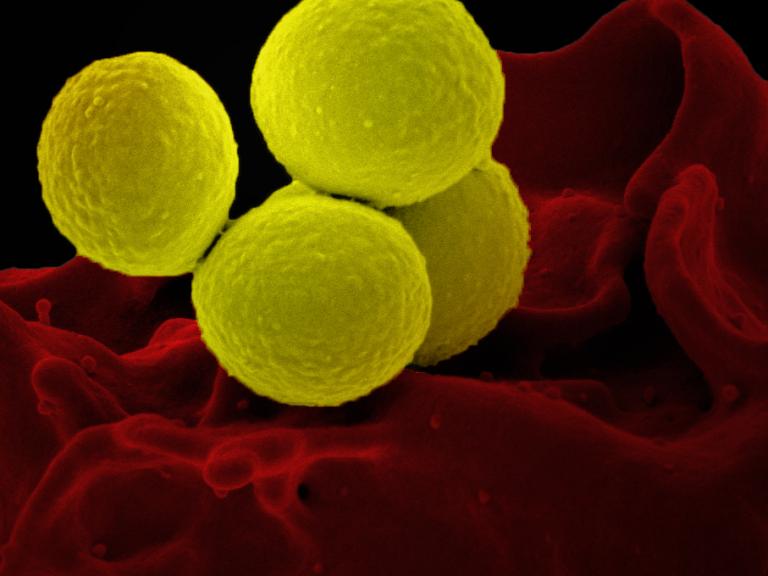
Trial of Existing Antibiotic for Treating Staphylococcus aureus Bacteremia Begins
The trial will test dalbavancin in 200 adults hospitalized with complicated S. aureus infection at approximately 20 trial sites around the United States.
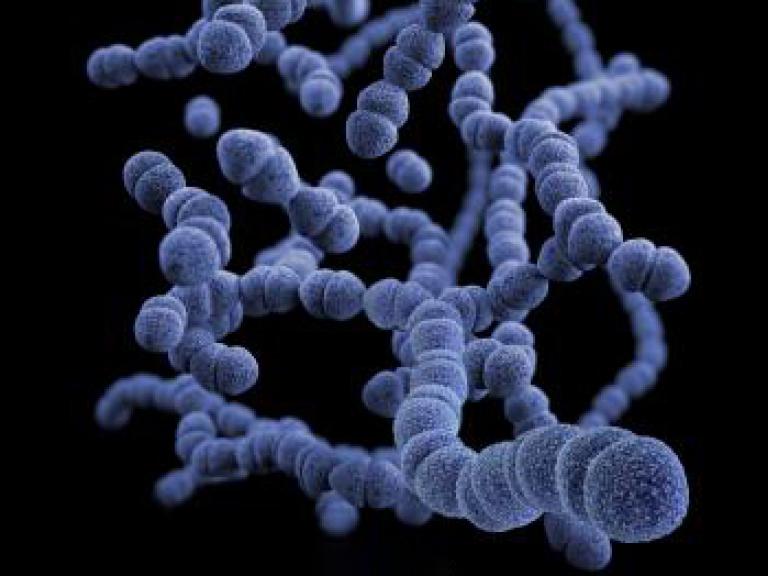
Childhood Pneumonia Study Shows Short-Course Antibiotics Superior to Standard of Care
A NIAID-sponsored randomized, double-blind, placebo-controlled trial study has shown that short-course (five day) antibiotic treatment is superior to standard (10 day) treatment of community-acquired pneumonia (CAP) in children age 6 months to 5 years.
Biocontainment Research Facilities
The National Biocontainment Laboratories (NBLs) and Regional Biocontainment Laboratories (RBLs) provide BSL4/3/2 and BSL3/2 biocontainment facilities, respectively, for research on biodefense and emerging infectious disease agents.
Investigators in academia, not-for-profit organizations, industry, and government studying biodefense and emerging infectious diseases may request the use of biocontainment laboratories. Please contact the NBLs and RBLs directly for further information.
Main Areas of Focus
- To conduct research on biodefense and emerging infectious disease agents.
- To be available and prepared to assist national, state, and local public health efforts in the event of a bioterrorism or infectious disease emergency.
Each NBL and RBL offers distinct resources for the research community. These include animal models, imaging services, and specialized equipment.
Please click on the links below for information about the services offered at each location.
Contact Information
Nancy Boyd, Section Chief, Extramural Biodefense Facilities, Office of Biodefense, Research Resources and Translational Research.
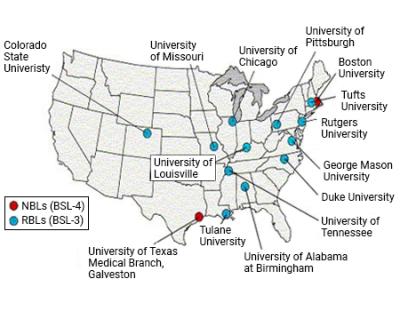
Map of NBL and RBL facility locations in the United States. The facilities are listed below.
National Biocontainment Laboratories
Regional Biocontainment Laboratories
Centers for Research on Structural Biology of Infectious Diseases (CRSTAL-ID)
The CRSTAL-ID program, formerly known as the Structural Genomics Centers, supports the characterization of the 3D structure of proteins of infectious disease pathogens. Each Center is a collaborative effort consisting of teams from multiple academic institutions/companies. Protein structures are requested by NIAID or the extramural research community and are made publicly available through online databases such as the Protein Data Bank. The structures help investigators better understand pathogen activity on a molecular level and assist in the identification of targets for drugs, vaccines, and other medical countermeasures.
Pandemic Preparedness Research Activities
Researchers funded through the CRSTAL-ID program have been at the forefront of basic and preclinical research activities for emerging infectious disease pathogens. For example, they have solved the structures of numerous SARS-CoV-2 proteins of high interest, including the spike protein, and have contributed substantially to the number of SARS-CoV-2 structures currently available in the Protein Data Bank. These efforts have provided critical insights into the molecular structure and mechanistic functions of SARS-CoV-2. In addition, CRSTAL-ID researchers have been active in efforts to identify and develop novel therapeutics, vaccines, and diagnostics for COVID-19. They have shown how different therapeutics bind to SARS-CoV-2 and have conducted preclinical screening to identify approved and candidate therapeutics with activity against SARS-CoV-2. They are using computational methods to facilitate the development of diagnostics and have been instrumental in structure-based vaccine design activities and efforts to determine correlates of protection for COVID-19.
Center Locations
Main Areas of Focus
The Centers provide the research community with
- Experimental 3-D protein structures and protein-ligand complexes (X-ray, NMR, CryoEM structures of NIAID category A-C priority agents and organisms causing emerging and re-emerging infectious diseases). 3-D structures and target lists can be found at:
- Sequence-verified clones, and peptides.
- Services that deliver requested 3-D structure determination.
- Molecular screening of proteins in complex with inhibitors, cofactors and substrate analogs
Contact Information
- Dr. Keehwan Kwon, Program Officer
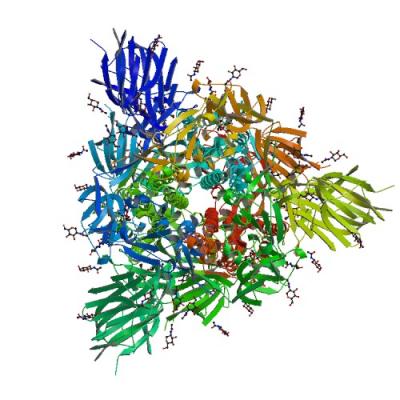
NIAID-funded investigators at the SSGCID determined the structure and function of the SARS-CoV-2 spike glycoprotein. The findings were made available in the Protein Data Bank and published in Cell.
- Tiny Nanoparticles Could Be A Big Jump for Flu Vaccines
- Scientists Take Aim at the Coronavirus Toolkit – Pacific Northwest National Laboratory
- New Drug Target Found for COVID-19 – Northwestern University Feinberg School of Medicine
- New coronavirus protein, mapped in Chicago, reveals drug target – University of Chicago
- Zeroing in on COVID-19 – Seattle Children’s Hospital Research Foundation
- COVID-19 coronavirus spike holds infectivity details – UW Medicine
- Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein
- De novo design of picomolar SARS-CoV-2 miniprotein inhibitors
- Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody
- Broad-spectrum inhibition of coronavirus main and papain-like proteases by HCV drugs
- Distinct B cell subsets give rise to antigen-specific antibody responses against SARS-CoV-2
- High-resolution structures of the SARS-CoV-2 2′-O-methyltransferase reveal strategies for structure-based inhibitor design
For more advances in research see CSGID Publications and SSGCID Publications.
STRucture-guided Influenza Vaccine InitiativE (STRIVE)
Vaccine and Treatment Evaluation Units (VTEUs) Services
The Vaccine and Treatment Evaluation Units (VTEUs), supported by the Division of Microbiology and Infectious Diseases (DMID) since the 1960s, provide a ready resource for the conduct of clinical trials to evaluate promising vaccines, treatments, and diagnostics for infectious diseases. The sites are part of DMID's Infectious Diseases Clinical Research Consortium (IDCRC).

