NIAID Scientists Detail First Structure of a Natural Mammalian Prion
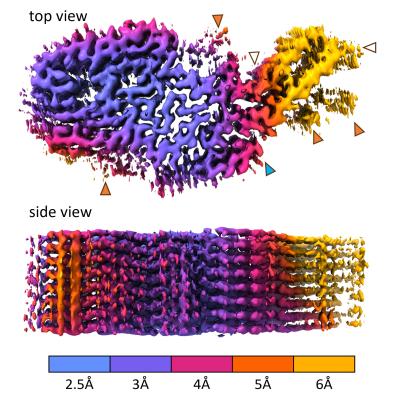
The near-atomic structure of a chronic wasting disease (CWD) prion should help scientists explain how CWD prions spread and become the most naturally infectious of the many mammalian protein aggregation diseases. NIAID scientists revealed the structure in a new study in Acta Neuropathologica. Such detailed knowledge could guide the rational design of vaccines and therapeutics, as well as identify mechanisms that protect humans from CWD pathogens in deer, elk, moose, and reindeer.
Many brain diseases of humans and other mammals involve specific proteins (e.g., prion protein or PrP) gathering into abnormal thread-like structures that grow by sticking to normal versions of the same protein. These threads can also fragment and spread throughout the nervous system and accumulate to deadly levels. For unknown reasons, CWD prions are more naturally contagious than most other protein aggregates and are spreading rampantly among cervid species in North America, Korea and northern Europe. Recalling the bovine spongiform encephalopathy (BSE) or “mad cow disease” epidemic of the mid-1980s and mid-1990s, there are concerns that CWD might similarly be transmissible to humans.
To date, no CWD transmission to humans has been substantiated, and the new CWD structure suggests preliminarily why we might be protected. The structure also reveals multiple differences between CWD and previously determined structures of highly infectious, but experimentally rodent-adapted, PrP-based prions. Differences are even more profound when compared to largely non-transmissible PrP filaments isolated from humans with Gerstmann-Sträussler-Scheinker syndrome, a genetic prion disorder.
PrP-based prion diseases are degenerative, untreatable, and fatal diseases of the central nervous system that occur in people and other mammals. These diseases primarily involve the brain, but also can affect the eyes and other organs. CWD-infected animals shed infectious prions in their feces, urine, and other fluids and body components while alive, and from their carcasses after dying. The prions can remain infectious in the environment for years.
Scientists at NIAID’s Rocky Mountain Laboratories in Hamilton, Montana, determined the CWD structure from the brain tissue from a naturally infected white-tailed deer. They isolated the prions and froze them in glass-like ice. Then, using electron microscopy techniques, they developed a 3-D electron density map that indicated the detailed shapes of the protein molecules within the prion structure. This involved taking nearly 80,000 video clips of the sample, magnified 105,000 times the original size, at various orientations. They marked prion filaments in the video clips and collected more than 500,000 overlapping sub-images. They isolated about 7,300 of the highest quality sub-images and then used supercomputers to generate a 3-D density map and a molecular model to fit the map.
Vaccine development is among the many research areas where scientists could use high-resolution prion structures to advance their work. The study authors note that previous attempts to develop vaccines against CWD in cervids failed to be protective, and, at least in one case, had the opposite effect. They speculate that one explanation for adverse vaccine effects could be that antibody binding to the sides, rather than the ends of prion fibril surfaces, promotes fragmentation – creating infectious particles rather destroying them. Thus, a strategy to explore with vaccines and small-molecule inhibitors, they say, is to target the tips of prion structures where binding and conversion of prion protein molecules occurs.
The research team is planning to solve other naturally occurring prion structures, hoping to advance its understanding of the molecular basis of prion transmission and disease.
References:
P Alam, F Hoyt, E Artikis, et al. Cryo-EM structure of a natural prion: chronic wasting disease fibrils from deer. Acta Neuropathologica DOI: 10.1007/s00401-024-02813-y (2024).
A Kraus et al. High-resolution structure and strain comparison of infectious mammalian prions. Molecular Cell. DOI: 10.1016/j.molcel.2021.08.011. (2021).