14 Results
Single Dose of Broadly Neutralizing Antibody Protects Macaques from H5N1 Influenza
February 11, 2025
A single dose of a broadly neutralizing antibody given prior to virus exposure protects macaques from severe H5N1 avian influenza, NIH scientists report.
New Antibodies Target “Dark Side” of Influenza Virus Protein
March 1, 2024
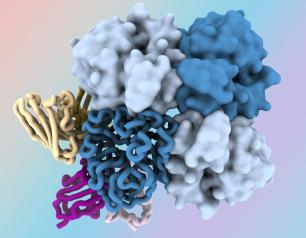
Researchers at the National Institutes of Health have identified antibodies targeting a hard-to-spot region of the influenza virus, shedding light on the relatively unexplored “dark side” of the neuraminidase (NA) protein head. The antibodies target a region of the NA protein that is common among many influenza viruses, including H3N2 subtype viruses, and could be a new target for countermeasures. The research, led by scientists at the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center, part of NIH, was published today in Immunity.
NIH-Developed HIV Antibodies Protect Animals in Proof-of-Concept Study
January 17, 2024
Three different HIV antibodies each independently protected monkeys from acquiring simian-HIV (SHIV) in a placebo-controlled proof-of-concept study intended to inform development of a preventive HIV vaccine for people. The antibodies—a human broadly neutralizing antibody and two antibodies isolated from previously vaccinated monkeys—target the fusion peptide, a site on an HIV surface protein that helps the virus fuse with and enter cells.
NIH Clinical Trial of Universal Flu Vaccine Candidate Begins
September 15, 2023
Enrollment in a Phase 1 trial of a new investigational universal influenza vaccine candidate has begun at the National Institutes of Health’s Clinical Center in Bethesda, Maryland. The trial is sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, and will evaluate the investigational vaccine for safety and its ability to elicit an immune response.
NIH Statement on HIV Vaccine Awareness Day 2023
May 18, 2023
Today marks the 26th observance of HIV Vaccine Awareness Day. The National Institutes of Health applauds the efforts of the collaborative global community of scientists, advocates, study participants, study staff, and funders enabling unprecedented levels of innovation and adaptation in the pursuit of a highly effective HIV vaccine.

Experimental Cancer Vaccine Shows Promise in Animal Studies
November 10, 2022
An experimental therapeutic cancer vaccine induced two distinct and desirable immune system responses that led to significant tumor regression in mice, report investigators from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.

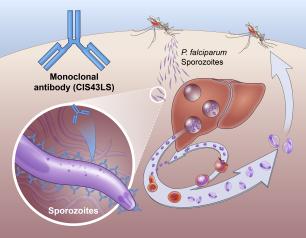
Monoclonal Antibody Prevents Malaria Infection in African Adults
October 31, 2022
One dose of an antibody drug safely protected healthy, non-pregnant adults from malaria infection during an intense six-month malaria season in Mali, Africa, a National Institutes of Health clinical trial has found. The antibody was up to 88.2% effective at preventing infection over a 24-week period, demonstrating for the first time that a monoclonal antibody can prevent malaria infection in an endemic region.
Vaccine for Rare but Deadly Mosquito-Borne Viruses Shows Promise in Clinical Trial
May 12, 2022
A vaccine for eastern equine encephalitis virus (EEEV), western equine encephalitis virus (WEEV), and Venezuelan equine encephalitis virus (VEEV) was found to be safe, well-tolerated and induced a neutralizing antibody response in adult volunteers, according to newly published results from a Phase 1 clinical trial.

Leadership Transition at the NIAID Vaccine Research Center
February 16, 2022
Dr. Fauci expresses gratitude to John R. Mascola, M.D., as he announces his retirement as Director of the Dale and Betty Bumpers Vaccine Research Center at the National Institute of Allergy and Infectious Diseases.
Experimental mRNA HIV Vaccine Safe, Shows Promise in Animals
December 9, 2021
An experimental HIV vaccine based on mRNA—the same platform technology used in two highly effective COVID-19 vaccines—shows promise in mice and non-human primates, according to scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health.
Monoclonal Antibody Prevents Malaria in Small NIH Trial
August 11, 2021
One dose of a new monoclonal antibody discovered and developed at the National Institutes of Health safely prevented malaria for up to nine months in people who were exposed to the malaria parasite. The small, carefully monitored clinical trial is the first to demonstrate that a monoclonal antibody can prevent malaria in people. The trial was sponsored and conducted by scientists from the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID), part of NIH, and was funded by NIAID.
NIH Launches Clinical Trial of Universal Influenza Vaccine Candidate
June 1, 2021
A first-in-human, Phase 1 trial assessing the safety and immunogenicity of an investigational nanoparticle influenza vaccine designed to provide long-lasting protection against multiple flu virus strains has begun at the National Institutes of Health Clinical Center in Bethesda, Maryland. Healthy participants 18 to 50 years old will receive either a licensed seasonal influenza vaccine or the experimental vaccine, FluMos-v1.
NIH Experts Discuss SARS-CoV-2 Viral Variants
February 12, 2021
The rise of several significant variants of SARS-CoV-2 has attracted the attention of health and science experts worldwide, NIH reports.
Media Availability—NIH Officials Highlight COVID-19 Vaccine Facts, Unknowns for Healthcare Providers
January 18, 2021
NIAID Director urges healthcare providers to be able to explain the latest data supporting the safety and efficacy of vaccines for COVID-19.

