131 Results
NIAID Selects Sarah Read as Principal Deputy Director
October 2, 2024
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH), has named Sarah W. Read, M.D., MHS, as the principal deputy director for the institute.

Clinical Trial of HIV Vaccine Begins in United States and South Africa
September 20, 2023
A trial of a preventive HIV vaccine candidate has begun enrollment in the United States and South Africa. The Phase 1 trial will evaluate a novel vaccine known as VIR-1388 for its safety and ability to induce an HIV-specific immune response in people.
U.S. Clinical Trials Begin for Twice-Yearly HIV Prevention Injection
June 4, 2024
Two clinical trials have launched to examine a novel long-acting form of HIV pre-exposure prophylaxis (PrEP) in cisgender women and people who inject drugs.
World TB Day 2024 – Yes! We Can End TB!
March 22, 2024
In observance of World Tuberculosis Day (Sunday, March 24), NIAID joins our partners in reaffirming our commitment to ending the tuberculosis (TB) pandemic while honoring the lives lost to TB disease.
NIH Launches Clinical Trial of Three mRNA HIV Vaccines
March 14, 2022
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has launched a Phase 1 clinical trial evaluating three experimental HIV vaccines based on a messenger RNA (mRNA) platform—a technology used in several approved COVID-19 vaccines. NIAID is sponsoring the study, called HVTN 302, and the NIAID-funded HIV Vaccine Trials Network (HVTN), based at Fred Hutchinson Cancer Research Center in Seattle, is conducting the trial.
NIH Experts Call for Accelerated Research to Address Concurrent HIV and COVID-19 Pandemics
April 8, 2021
The COVID-19 pandemic is affecting people with or at risk for HIV both indirectly, by interfering with HIV treatment and prevention services, and directly, by threatening individual health. An effective response to these dual pandemics requires unprecedented collaboration to accelerate basic and clinical research, as well as implementation science to expeditiously introduce evidence-based strategies into real-world settings. This message comes from a review article co-authored by Anthony S.
NIH Statement on HIV Vaccine Awareness Day 2023
May 18, 2023
Today marks the 26th observance of HIV Vaccine Awareness Day. The National Institutes of Health applauds the efforts of the collaborative global community of scientists, advocates, study participants, study staff, and funders enabling unprecedented levels of innovation and adaptation in the pursuit of a highly effective HIV vaccine.

NIH Researchers Identify How Two People Controlled HIV After Stopping Treatment
October 28, 2021
Research led by scientists at the National Institutes of Health has identified two distinct ways that people with HIV can control the virus for an extended period after stopping antiretroviral therapy (ART) under medical supervision. This information could inform efforts to develop new tools to help people with HIV put the virus into remission without taking lifelong medication, which can have long-term side-effects.
Antibody Infusions Prevent Acquisition of Some HIV Strains, NIH Studies Find
January 26, 2021
NIH finds that an investigational anti-HIV antibody prevented acquisition of some HIV strains, but did not significantly reduce overall acquisition.
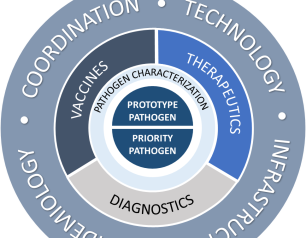
NIAID Pandemic Preparedness Plan Targets ‘Prototype’ and Priority Pathogens
February 2, 2022
The National Institute of Allergy and Infectious Diseases is focusing on preparing for a range of other viral threats that could cause a public health emergency, and according to NIAID’s new Pandemic Preparedness Plan, the institute will direct its preparedness efforts on two fronts.
NIH Experts Review Monkeypox Challenges
August 24, 2022
National Institutes of Health experts write that lessons learned from the public health responses to the HIV and COVID-19 pandemics should help guide the response to the current outbreak of monkeypox.
Dr. Fauci Reflects on the Perpetual Challenge of Infectious Diseases
November 28, 2022
Dr. Fauci, who since 1984 has directed the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, reflects on his career responding to infectious disease threats.

U.S. Clinical Trial Evaluating Antiviral for Monkeypox Begins
September 9, 2022
A Phase 3 clinical trial evaluating the antiviral tecovirimat, also known as TPOXX, is now enrolling adults and children with monkeypox infection in the United States. Study investigators aim to enroll more than 500 people from clinical research sites nationwide. Interested volunteers can visit the ACTG website (clinical trial A5418) for more information. The NIAID-funded Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) is leading the study, which may later expand to international sites. The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) at NIH is supporting several sites, including through the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT).
NIAID Appoints Ted Pierson as New Vaccine Research Center Director
April 25, 2023
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has named Theodore (Ted) C. Pierson, Ph.D., as the new director of its Dale and Betty Bumpers Vaccine Research Center (VRC) in Bethesda, MD.

Statement by Anthony S. Fauci, M.D.
August 22, 2022
I am announcing today that I will be stepping down from the positions of Director of the National Institute of Allergy and Infectious Diseases (NIAID) and Chief of the NIAID Laboratory of Immunoregulation, as well as the position of Chief Medical Advisor to President Joe Biden. I will be leaving these positions in December of this year to pursue the next chapter of my career.
NIH Scientists Identify Nutrient that Helps Prevent Bacterial Infection
January 15, 2021
NIAID scientists studying natural defenses against bacterial infection found that taurine helps the gut recall prior infections and kill invading bacteria.
Monkeypox Treatment Trial Begins in the Democratic Republic of the Congo
October 12, 2022
A clinical trial to evaluate the antiviral drug tecovirimat, also known as TPOXX, in adults and children with monkeypox has begun in the Democratic Republic of the Congo (DRC). The trial will evaluate the drug’s safety and its ability to mitigate monkeypox symptoms and prevent serious outcomes, including death. NIAID and the DRC’s National Institute for Biomedical Research (INRB) are co-leading the trial as part of the government-to-government PALM partnership. Collaborating institutions include the U.S. Centers for Disease Control and Prevention (CDC), the Institute of Tropical Medicine Antwerp, the aid organization Alliance for International Medical Action (ALIMA) and the World Health Organization (WHO).
World TB Day 2022—Invest to End TB. Save Lives
March 24, 2022
Today marks the 140th anniversary of the announcement by Dr. Robert Koch that tuberculosis (TB) is caused by the bacterium Mycobacterium tuberculosis. World TB Day is a reminder that this ancient disease remains a relentless killer. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, affirms its commitment to the 2022 World TB Day theme, Invest to End TB. Save Lives, by supporting and conducting wide-ranging research aimed at reducing the health and economic impacts of TB.
Statement—Large Clinical Trial Will Test Combination Monoclonal Antibody Therapy for Mild/Moderate COVID-19
January 5, 2021
A NIAID-supported clinical trial has begun to evaluate a combination investigational monoclonal antibody therapy for people with mild to moderate COVID-19.
NIH Research Identifies Opportunities to Improve Future HIV Vaccine Candidates
December 14, 2023
An effective HIV vaccine may need to prompt strong responses from immune cells called CD8+ T cells to protect people from acquiring HIV, according to a new study from researchers at the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, and colleagues. The study findings, appearing in Science, draw comparisons between the immune system activity of past HIV vaccine study participants and people with HIV who naturally keep the virus from replicating even in the absence of antiretroviral therapy (ART).
Investigational Three-Month TB Regimen Is Safe but Ineffective, NIH Study Finds
July 5, 2023
The first clinical trial of a three-month tuberculosis (TB) treatment regimen is closing enrollment because of a high rate of unfavorable outcomes with the investigational course of treatment. Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG) 5362, also known as the CLO-FAST trial, sought to evaluate the safety and efficacy of a three-month clofazimine- and high-dose rifapentine-containing regimen. An interim data analysis showed that participants taking the investigational regimen experienced ongoing or recurring TB at rates above thresholds set in the study protocol.
Combination Anti-HIV Antibody Infusions Suppress Virus for Prolonged Period
June 1, 2022
According to a small study published today in the journal Nature, individuals with HIV who began taking antiretroviral therapy (ART) in the early stages of infection achieved a lengthy period of HIV suppression without ART after receiving two broadly neutralizing anti-HIV antibodies (bNAbs).
Statement—Four Potential COVID-19 Therapeutics Enter Phase 2/3 Testing in NIH ACTIV-2 Trial
February 12, 2021
Enrollment has begun to test additional investigational drugs in the NIH Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program.
NIH Researchers Discover Novel Class of Anti-Malaria Antibodies
January 3, 2025
New antibodies that bind to a previously untargeted portion of the malaria parasite could lead to new monoclonal antibody treatments and vaccines for malaria.
Tecovirimat Is Safe but Ineffective as Treatment for Clade II Mpox
March 12, 2025
Monotherapy with the antiviral drug tecovirimat was safe but ineffective as an mpox treatment in an international clinical trial.