November 21, 2022
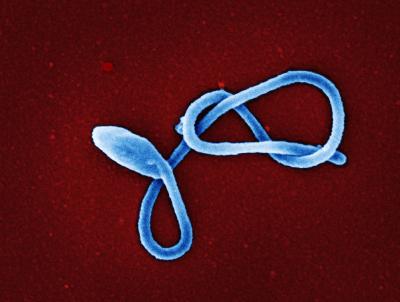
Colorized scanning electron micrograph of a single filamentous Ebola virus particle (colorized blue). Image captured and color-enhanced at the NIAID Integrated Research Facility in Ft. Detrick, Maryland.
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, recently awarded more than $12 million to three institutions for the development of antiviral therapies to treat diseases caused by viruses with pandemic potential. NIAID may award approximately $61.5 million total over five years if all contract options are exercised. The new product development contracts are part of the Antiviral Program for Pandemics (APP), which aims to accelerate the discovery, development and manufacturing of antiviral medicines.
Antivirals are treatments that fight viral infections by acting directly against the virus. Other types of therapies, not the focus of this program, harness the body’s immune system to fight infection. The new contracts will support the development of promising antiviral candidates from late-stage preclinical studies through investigational new drug application-enabling activities and clinical testing.
Alongside the new product development contracts, NIAID already supports nine Antiviral Drug Discovery (AViDD) Centers for Pathogens of Pandemic Concern. The AViDD Centers conduct research on the early-stage identification and validation of novel viral targets and identification and early-stage characterization of antiviral drug candidates.
The new product development contracts include:
Optimization of Broad-Spectrum Filovirus Inhibitors that Target Viral Glycoprotein
Principal investigator: Terry Bowlin, Ph.D.
Institute: Microbiotix, Inc., Worcester, Massachusetts
Base funding amount: $2,069,416
NIAID contract: 75N93023C00001
Development of a Novel 2-Pyrimidone (SRI-42718) as a Potent Inhibitor of Chikungunya Virus Infection and Disease
Principal investigator: Daniel Streblow, Ph.D.
Institute: Oregon Health and Science University, Portland
Base funding amount: $4,696,452
NIAID contract: 75N93023C00002
Development of an Orally Available Antiviral Drug for Yellow Fever
Principal investigator: Jinhong Chang, M.D., Ph.D.
Institute: Baruch S. Blumberg Institute, Doylestown, Pennsylvania
Base funding amount: $5,493,876
NIAID contract: 75N93023C00003
For more information about the APP, please visit: https://www.niaid.nih.gov/research/antivirals.
WHO:
Emily Erbelding, M.D., M.P.H., director of NIAID’s Division of Microbiology and Infectious Diseases, is available for comment.
Contact
Submit a Media Request
Contact the NIAID News & Science Writing Branch.

