Study Aims to Determine Safety and Efficacy of Experimental Monoclonal Antibodies
August 4, 2020
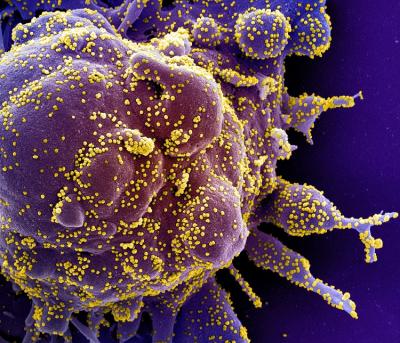
Colorized scanning electron micrograph of a cell (purple) heavily infected with SARS-COV-2 virus particles (yellow), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland.
Patients admitted with COVID-19 at select hospitals may now volunteer to enroll in a clinical trial to test the safety and efficacy of a potential new treatment for the disease. The Phase 3 randomized, controlled trial is known as ACTIV-3, and as a “master protocol,” it is designed to expand to test multiple different kinds of monoclonal antibody treatments. It also can enroll additional volunteers in the middle of the trial, if a specific investigational treatment shows promise.
The new study is one of four ongoing or planned trials in the National Institutes of Health’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program, a public-private partnership to speed development of the most promising treatments and vaccine candidates. It also is receiving support through Operation Warp Speed, the U.S. government’s multi-agency effort to develop, manufacture and distribute medical countermeasures to fight COVID-19.
The trial will take place at select hospitals around the world that are part of existing clinical trial networks. They include the lead network, the International Network of Strategic Initiatives in Global HIV Trials (INSIGHT), operated by the National Institute of Allergy and Infectious Diseases (NIAID), a part of the National Institutes of Health. Collaborating clinical trial networks include the Prevention and Early Treatment of Acute Lung Injury network (PETAL) and Cardiothoracic Surgical Trials Network (CTSN), supported by the NIH’s National Heart, Lung and Blood Institute through the Collaborating Network of Networks for Evaluating COVID-19 and Therapeutic Strategies (CONNECTS) program, and the U.S. Department of Veterans Affairs Medical Centers.
“Under Operation Warp Speed, the U.S. Government has brought together multiple agencies to accelerate the development, manufacture and distribution of medical countermeasures for COVID-19,” said NIH Director Francis S. Collins, M.D., Ph.D. “The ACTIV studies are just a few examples of this critical activity, which emphasizes flexibility and minimizes delays to generate scientifically sound results.”
ACTIV-3 uses an adaptive two-stage Phase 3 protocol design. The ACTIV-3 trial can be modified to test additional experimental therapeutics and flexibly allow novel therapeutics to enter at either stage 1 or stage 2. In addition, if a treatment appears to be safe and effective in the initial stage after review by an independent data and safety monitoring board (DSMB), the investigational therapeutic proceeds to stage 2 testing, where more volunteers are enrolled. If an investigational therapeutic is unsafe or not likely to be effective, it will be dropped.
The ACTIV-3 study will begin by studying the investigational monoclonal antibody LY-CoV555, which was identified in a blood sample from a recovered COVID-19 patient. Antibodies are infection-fighting proteins made by the immune system that can bind to the surface of viruses and prevent them from infecting cells. Synthetic versions of antibodies can be reproduced in a laboratory. These manufactured antibodies are known as monoclonal antibodies. The LY-CoV555 antibody was discovered by AbCellera Biologics (Vancouver, British Columbia) in collaboration with NIAID’s Vaccine Research Center. Subsequently, it was developed and manufactured by Lilly Research Laboratories, Eli Lilly and Company (Indianapolis, Indiana), in partnership with AbCellera. The investigational product also is being tested in another ongoing NIAID study, ACTIV-2, which is studying its safety and efficacy in people with mild to moderate symptoms of COVID-19 who have not been hospitalized. Safety data and other findings will be shared across the ACTIV-2 and ACTIV-3 studies through the DSMB.
“Studying the impact of this investigational therapeutic on multiple patient populations at the same time is critical to determining whether it can help COVID-19 patients with differing levels of disease severity,” said NIAID Director Anthony S. Fauci, M.D. “These concurrent trials have the potential to yield significant and comprehensive clinical data.”
The initial stage of the ACTIV-3 clinical trial aims to enroll approximately 300 volunteers who have been hospitalized with mild to moderate COVID-19 with fewer than 13 days of symptoms. Once their COVID-19 infections have been confirmed and they have consented to take part in the study, participants will be randomly assigned to receive either an intravenous (IV) infusion of LY-CoV555 or a saline placebo infusion. Participants also will receive standard care for COVID-19, including the antiviral remdesivir. After five days, participants’ symptoms will be assessed, as will their need for supplemental oxygen, mechanical ventilation, or other supportive care. Volunteers will be followed for 90 days after enrollment and will receive regular examinations and have blood samples taken periodically during this time to analyze their response to the investivational therapeutic.
Data collected on the fifth day of the volunteers’ participation will determine whether the investigational therapeutic will be administered to a larger group of volunteers. If LY-CoV555 appears to be safe and appears to be effective, the trial will enroll an additional 700 participants. It also will begin enrolling more severely ill participants, such as those with organ failure requiring mechanical support, or COVID-19-associated dysfunction of organs other than the lungs. The primary endpoint of the trial is the participants’ sustained recovery for 14 days after release from the hospital.
The principal investigator of ACTIV-3 is Jens Lundgren, M.D., of the University of Copenhagen and Rigshospitalet. Leads of the participating networks include James Neaton, Ph.D., of the INSIGHT network, Taylor Thompson, M.D., of the PETAL network, Annetine Gelijns, Ph.D., and Alan Moskowitz, M.D., of the CTSN, and Rachel Ramoni, D.M.D., Sc.D., of the U.S. Department of Veterans Affairs. To ensure that the trial is being conducted in a safe and effective manner, an independent DSMB will oversee the trial and conduct periodic reviews of the accumulating data.
Contact
To schedule interviews, contact:
Elizabeth Deatrick
(301) 402-1663
NIAIDNews@niaid.nih.gov
Questions & Answers
ACTIV-3 Clinical Trial to Evaluate Monoclonal Antibodies and Other Potential Treatments in Hospitalized COVID-19 Patients
This clinical trial, called ACTIV-3, will evaluate the safety and efficacy of investigational therapeutics as a treatment for participants hospitalized with COVID-19. The multicenter, adaptive Phase 3 clinical trial will begin by evaluating LY-CoV555, an experimental monoclonal antibody treatment made by Lilly Research Laboratories, Eli Lilly and Company (based in Indianapolis), in partnership with AbCellera Biologics (Vancouver, British Columbia). AbCellera and scientists at the National Institute of Allergy and Infectious Diseases (NIAID), part of the U.S. National Institutes of Health, isolated the LY-CoV555 antibody from a blood sample from a patient who recovered from a confirmed case of COVID-19. The antibody was copied and then synthesized in a laboratory—the term “monoclonal” refers to these laboratory-manufactured versions of one exact antibody naturally produced by the immune system in response to invading viruses or other pathogens. The investigational product is also being tested in non-hospitalized people with symptoms from COVID-19 in a clinical trial called ACTIV-2.
ACTIV-3 uses an adaptive two-stage Phase 3 protocol design. The ACTIV-3 trial can be modified to test additional experimental therapeutics and flexibly allow novel therapeutics to enter at either stage 1 or stage 2 depending on prior knowledge of activity and safety. If a previously limited tested treatment appears to be safe and have activity in the initial stage 1 after review by an independent data and safety monitoring board (DSMB), the investigational therapeutic proceeds to stage 2 testing, where more volunteers are enrolled in order to clarify whether it is effective and safe. If an investigational therapeutic fails to show safety and activity in stage 1, it will be dropped.
The trial will begin by evaluating the LY-CoV555 monoclonal antibody but is designed to test other investigational therapeutics as they become available.
NIAID is the regulatory sponsor and holder of the investigational drug application to conduct the ACTIV-3 study. The trial is being funded by NIAID through “Operation Warp Speed,” a partnership led by the U.S. Department of Health and Human Services to invest in and coordinate the development, manufacturing and distribution of COVID-19 diagnostics, therapeutics, and vaccines.
The clinical trial is one of four ongoing or planned trials in NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program, a public-private partnership or initiative to speed development of the most promising treatments and vaccine candidates.
The ACTIV-3 study will be led by principal investigator Jens Lundgren, M.D., DMSc, of the University of Copenhagen and Rigshospitalet. The NIAID-funded International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) will lead the trial in collaboration with the Prevention and Early Treatment of Acute Lung Injury network (PETAL) and Cardiothoracic Surgical Trials Network (CTSN), both supported by the NIH’s National Heart, Lung and Blood Institute and the U.S. Department of Veterans Affairs (VA) Medical Centers.
Leads of the participating networks include James Neaton, Ph.D., of the INSIGHT network, Taylor Thompson, M.D., of the PETAL network, Annetine Gelijns, Ph.D., and Alan Moskowitz, M.D. of the CTSN, and Rachel Ramoni, D.M.D., Sc.D., of the U.S. VA.
The trial will take place in at least 100 select major medical centers in the United States and around the world participating in the INSIGHT, PETAL, CTSN and VA networks.
ACTIV-3 uses an adaptive two-stage Phase 3 protocol design, which can be modified to test additional experimental therapeutics and flexibly allow novel therapeutics to enter at either stage 1 or stage 2. If a treatment appears to be safe and active in the initial stage 1 as determined by an independent data and safety monitoring board (DSMB), the investigational therapeutic proceeds to stage 2 testing, where more volunteers are enrolled in order to determine its efficacy and safety. If an investigational therapeutic fails to show safety and activity in stage 1, it will be dropped.
In stage 1, the study aims to enroll 300 inpatient volunteers who are at least 18 years or older who have had COVID-19 symptoms for 12 days or less and without end-stage organ failure. Participants will be randomly assigned to receive either the investigational agent (150 participants) or placebo (150 participants). After five days, participants’ symptoms will be assessed, as will their need for supplemental oxygen, mechanical ventilation or other supportive care. If day 5 data appear favourable, Stage 2 of the study will begin enrolling an additional 700 volunteers with the same criteria except may also include patients with other types of end-stage organ dysfunctions or failure.
All study participants will receive COVID-19 standard of care, including treatment with the antiviral drug remdesivir and possible dexamethasone. All volunteers will be followed for 90 days after enrollment and receive clinical examinations and have periodic blood samples taken to analyze their response to the experimental therapeutic.
Careful safety assessments are done throughout the trial. An independent data and safety monitoring board (DSMB) will regularly review the clinical trial’s safety and efficacy data and use established guidelines to determine whether the investigational product should advance from stage 1 to stage 2. The DSMB can recommend that NIH terminate the study at any time if deemed necessary for safety reasons. The DSMB for this trial is a multi-disciplinary team. A central investigational review board will also oversee participant safety.
The primary goal of the ACTIV-3 clinical trial is the participant’s sustained recovery for 14 days at home after hospital discharge.
ACTIV 3 study sites are located in hospitals throughout the country, including VA hospitals as well as in several other countries around the world. The diversity of populations served by these hospitals will help ensure the trial enrolls those at greatest risk from COVID-19.
ACTIV 3 will be an ongoing study, eventually including several additional investigational monoclonal antibodies and therapeutics. It is anticipated that results from the first product may be available in the fall of 2020.
The study is designed to add other investigational treatments as they become available. Each new investigational therapeutic will be compared to a shared placebo group.

