Credit:
NIAID
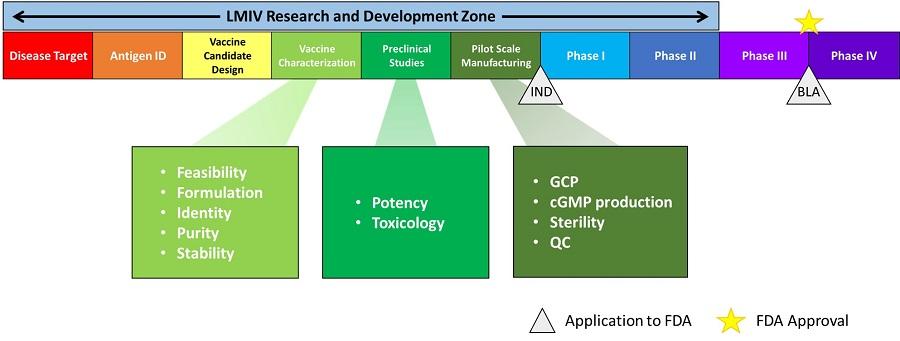
Vaccine candidates move through the following stages of development as they are tested for safety and efficacy:
- Disease target is identified
- Antigen is identified
- Vaccine candidate is designed
- Vaccine is characterized according the following criteria:
- Feasibility
- Formulation
- Identity
- Purity
- Stability
- Preclinical studies begin, testing for the following:
- Potency
- Toxicology
- Manufacturing begins on a pilot scale, where the candidate is evaluated according to the following controls:
- Good Clinical Practices
- Clinical Good Manufacturing Practices (cGMP)
- Sterility
- Quality control
- Investigational New Drug (IND) application is filed with the FDA
- Phase I clinical testing begins
- Phase II clinical testing begins
- Phase III clinical testing begins
- Biologics License Application (BLA) application is filed with the FDA
- FDA approves the candidate
- Phase IV clinical testing begins
LMIV research and development engages in Steps 1 through 9, working with collaborators who, if applicable, see candidates through the final steps.
Very few vaccines successfully complete all of these steps, as they are intended to ensure that only the safest, most effective drugs reach the market.

