- Because the research will not involve standard staining panels or data processing protocols until substantial experience with diverse projects has been accumulated, it is essential to choose initial projects based both on feasibility with current methods and the underlying scientific merit of the study itself. These variables also limit a fully accurate estimation of how many projects of what scope can be undertaken during the early (first year) phase of the CAT-I’s operation.
- Given existing resources, Figure 1 summarizes the estimated time to acquire and process each section using an established staining panel of 8 to 12 colors. Creation of new panels will vary in time to development depending on whether they are a combination of validated antibodies and fluorochromes (a few weeks) or involve novel reagents (1 to 2 months depending on whether the target antigen requires antigen recovery method development for formalin-fixed, paraffin-embedded (FFPE) material, availability or ease of generating new fluorochromes conjugates, and so on).
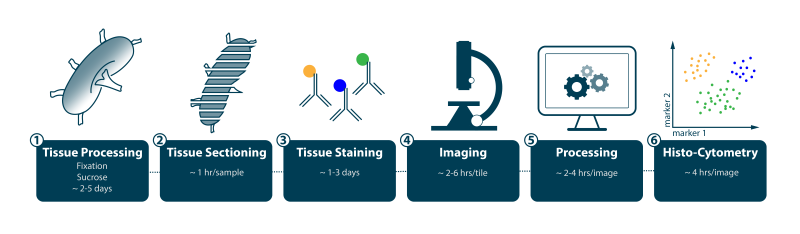
Histo-cytometry workflow. Estimated time to acquire and process each section using an established staining panel of 8 to 12 colors.
Credit: Stefan Uderhardt, LSB, NIAID
Imaging using the newly developed clearing and quantitative imaging method of Ce3D has much greater time demands due to the various steps in getting full penetration of a multi-mm-thick sample with conjugated antibodies (several days to 2 weeks), performing the clearing step (several days), and imaging the much larger sample at high resolution (as long as 18 to 24 hours). In addition, imaging at greater than 600 µm depth requires a special instrument different from the two main production microscopes that will be used for imaging of < 30 μM sections. For these reasons, use of this method will be limited to carefully selected samples or projects determined in consultation with the CAT-I staff. - Based on the assumption that for section-based analyses each sample will require as many as 6 sections to provide adequate coverage of a small block of complex tissue, data acquisition for studies with 10 samples (~3 mm3) should be completed in 2 weeks. First-pass analysis for qualitative image generation would then take 2 weeks. Detailed quantitative analysis production will vary with the nature of the sample and the information needed by the investigator but typically takes 4 hours per section when conducted by a trained expert.
- During its first year of operation, investigators in the NCI Center for Cancer Research (CCR), NIAID Division of Intramural Research (DIR), and selected extramural investigators working in the areas of immunotherapy trials for cancer, infectious disease, or autoimmunity will be encouraged to develop short proposals for imaging-based studies to be conducted in collaboration with the CAT-I. Online resources are available on this site, —listing target antigens the CAT-I can confidently detect in optimally fixed or FFPE material as well as examples of lymphoid, myeloid, and stromal antigen panels the CAT-I has pre-tested—to guide investigators in designing potential studies that can be conducted quickly because new panel development will be held to a minimum. These proposals will be reviewed by a scientific advisory board consisting of two NIAID investigators, two NCI investigators, one extramural imaging expert, and the CAT-I director.
Proposals will be evaluated for technical feasibility (number of samples, availability of adequate tissue, conditions of tissue collection, planned use of established reagents and panels) and for scientific merit that takes NIAID and NCI priorities into account. The director will consult with CAT-I staff as needed during these evaluations to ensure that planned studies have a high likelihood of successful completion in a reasonably predictable time frame. The highest-ranked proposals will be advanced to the next stage of collaborative discussions between CAT-I staff and the investigator, to ensure that the anticipated contribution of the imaging analysis to the overall project is well-defined, that there is a clear understanding of what is required of the investigator to ensure the likely production of high-quality data, that the time frame for data acquisition and for data analysis is understood, that there is an agreed-upon plan for eventual publication of the findings, and to determine the possible involvement of staff from the proposing investigator’s laboratory in staining panel development and data analysis steps. To help increase throughput, it will be very useful to train non-CAT-I staff to help conduct analysis in studies involving a substantial number of samples and to provide these individuals the needed computational facilities, so that the CAT-I staff can devote themselves to projects where such external staff is either unavailable or unnecessary.
- Studies in which quantitative imaging analysis is a crucial component will be given priority over proposals to just ‘get a picture’ to put into a paper focused on other aspects of biology. However, in some cases where acquisition of a few images could add great value to an otherwise important study, these proposals will be advanced and, when possible, the imaging conducted quickly so that submission for publication can proceed without delay.
- Based on all these considerations, the CAT-I will establish a timeline for project initiation and completion in coordination with the project’s principal investigator. CAT-I will also fit these timelines together into a larger plan of operation covering at least a 6-month, forward-looking time frame.
- Initial image data will be processed to remove artifacts, deal with channel spillover, and eliminate background autofluorescence, producing a primary qualitative dataset that the investigator can peruse; this can be valuable because in many instances, biologically relevant patterns of tissue architecture and cell distribution are clearly visible without full quantitative Histo-cytometric processing of the data. The CAT-I staff and the investigators will then discuss these initial data and devise a plan for secondary, quantitative analysis as needed for the project goals.
- Once the primary and secondary data analyses are complete, the CAT-I staff and director will discuss with the investigator the interpretation of the data and work with the investigator on how the findings can best be reported.
Although the interactions of the CAT-I with investigators are considered collaborative, the CAT-I staff and the director understand that the projects will have originated with the CCR, DIR, or extramural investigator, that each project may be part of an ongoing series of studies on a topic that is the core focus of the laboratory proposing the study, and that papers resulting from interactions with CAT-I often are critical to the career of trainees or junior faculty involved in such studies. Therefore, the CAT-I staff and the director have already agreed internally that as a general policy, it will be presumed that the first and last listed authors of collaborative papers will be members of the outside laboratory proposing the project, subject to discussion. In some cases, co-first or -last authorship by CAT-I staff may be appropriate, but such designations will be determined by discussions with the collaborating investigator.
- Because even under optimal circumstances, CAT-I will not be able to handle all the requests for collaborations that have scientific merit, CAT-I plans to hold one or more courses in Histo-cytometry and Ce3D methods each year, which would include training in panel development, staining, data acquisition, and data processing using the methods developed in the LBS. It would be best to have participants from core imaging facilities attend, so that the methods can serve the largest number of individuals at the home institution or ICD, but if an investigator is planning to use these methods on an ongoing basis for large studies, members of a particular laboratory would also be considered as possible students.
Research
Research Areas
Grants & Contracts
Clinical Trials
News & Events
About NIAID
Website Policies and Notices

