Contact
Submit a Media Request
Contact the NIAID News & Science Writing Branch.


Submit a Media Request
Contact the NIAID News & Science Writing Branch.
The Chemistry Center for Combating Antibiotic-Resistant Bacteria (CC4CARB) is a NIAID-led partnership with RTI International, which oversees the design, synthesis, and management of external investigator-submitted libraries of chemical compounds specifically targeting Gram-negative bacteria. Resulting CC4CARB compounds subsequently will be publicly disclosed and freely available to researchers around the world for use in new research.
The SARS-CoV-2 Interagency Group (SIG), established by the U.S. Department of Health and Human Services (HHS), works to rapidly characterize emerging variants and actively monitors their potential impact on SARS-CoV-2 vaccines, therapeutics, and diagnostics. The SIG is responsible for variant classifications in the United States and meets regularly to evaluate the risk posed by SARS-CoV-2 variants circulating in the United States and globally to make recommendations about the variants. NIAID is a member of the SIG and supports research and surveillance activities to help inform decisions made by the SIG.
To support the generation of data for the SIG, NIAID has created the SARS-CoV-2 Assessment of Viral Evolution (SAVE) risk-assessment pipeline for SARS-CoV-2 variant viruses. SAVE provides a comprehensive real-time risk assessment of emerging mutations in SARS-CoV-2 that could impact transmissibility, virulence, and susceptibility to infection- or vaccine-induced immunity. The goals of the SAVE program are to understand emerging changes in the virus that could impact transmissibility, virulence, susceptibility to convalescent and vaccine-induced immunity and diagnostic testing. SAVE is one of the data streams that provides important information for variant characterization and complements other USG and NIH efforts like the ACTIV Tracking Resistance and Coronavirus Evolution (TRACE) initiative.
The NIAID SAVE program is composed of an international team of scientists with expertise in virology, immunology, vaccinology, structural biology, bioinformatics, viral genetics, and evolution. Researchers from NIAID Intramural, the NIAID Vaccine Research Center, other HHS and Department of Defense laboratories, and the extramural academic community work collaboratively within and across multiple sub-groups to accelerate the pace of variant research and discovery through rapid and open sharing. Researchers are developing high quality data sets that are then vetted by SIG working groups comprised of various experts. These data help inform public health recommendations.
More information on the SAVE program can be found in the publication, "Defining the risk of SARS-CoV-2 variants on immune protection" (Nature, 2022).
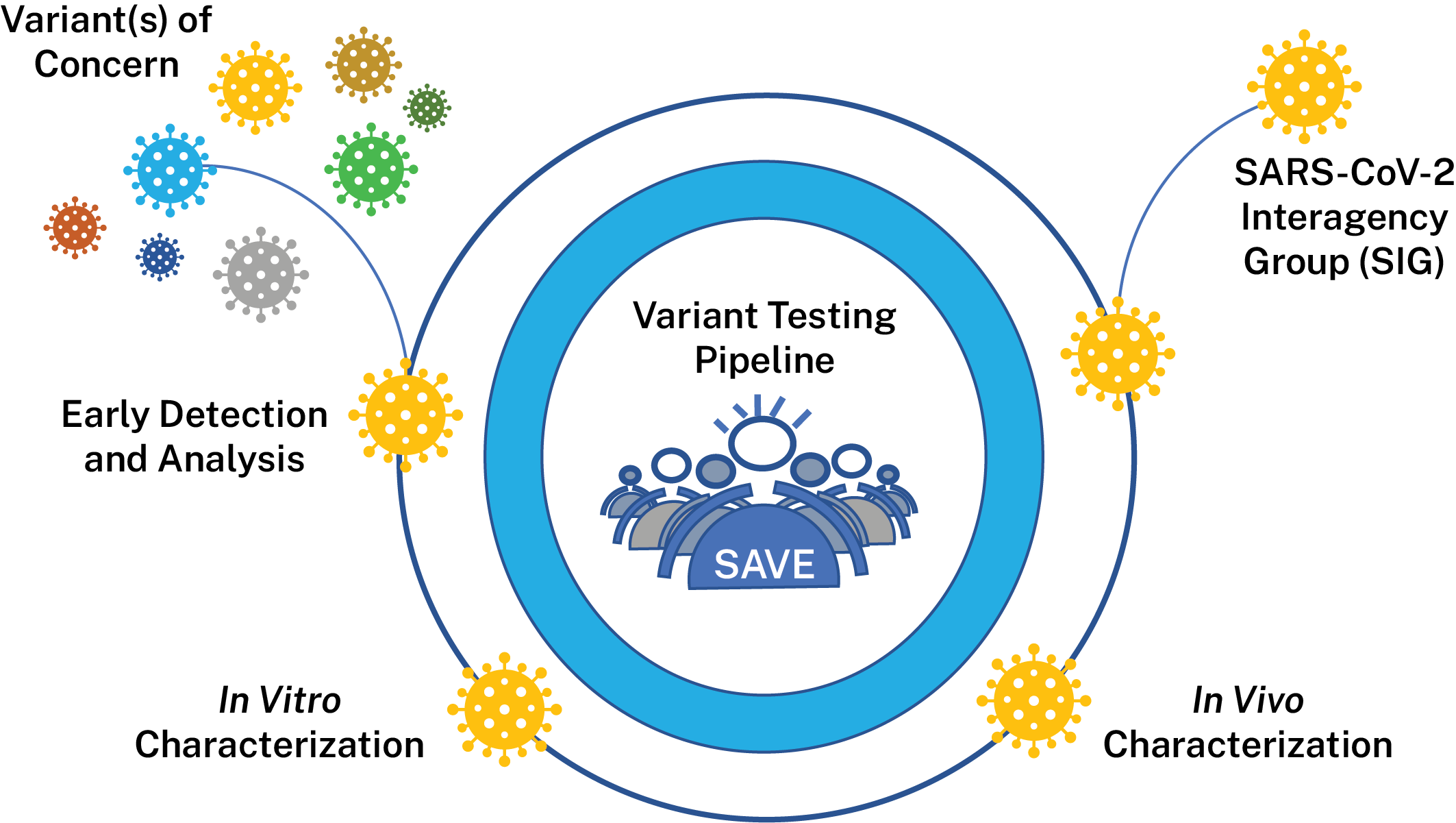
NIAID’s activities are divided into the following data-generating components:
Lead: Elodie Ghedin, Ph.D., NIAID and Derek Smith, Ph.D., Cambridge University
This team is evaluating surveillance/epidemiological data, information from observational studies on the efficacy of COVID-19 vaccines, variant information from documented vaccine breakthrough cases and accompanying sequence data from these efforts to identify variants that should be evaluated further in vitro and in vivo. They will also perform antibody escape mapping in the lab using monoclonal antibodies and polyclonal sera in pseudoviral assays to identify proactively substitutions of concern.
Lead: Florian Krammer, Ph.D., Icahn School of Medicine at Mount Sinai and Mehul Suthar, Ph. D., Emory University
This team characterizes variants identified by the early detection group comprehensively in vitro to determine how they may differ from the original SARS-CoV-2 virus. Variant viruses, pseudoviruses and various convalescent and vaccinee sera samples are used to determine if variants are able to evade infection- or vaccine-induced immunity. Groups are also performing antigenic landscape analyses to determine the relatedness of each emerging variant to the original strain to which vaccines were made. Researchers also analyze characteristics such as in vitro replication kinetics to determine how variant viruses may have different characteristics that affect their ability to replicate or transmit between humans. Groups are also exploring if any variants can evade cellular immune responses generated by memory B-cells and T-cells. The data generated by this groups helps determine which variants are of most concern for our existing countermeasures.
Lead: Michael Diamond, M.D., Ph.D., and Jacco Boon, Ph.D., Washington University in St. Louis
This team is using animal models to estimate virulence changes in circulating SARS-CoV-2 viruses and evaluate vaccine efficacy against variant viruses. Researchers use various animal models to examine if any variants cause different levels of disease in models. Researchers also vaccinate or infect animals to determine if animals remain protected from infection with variant viruses over different periods of time. Finally, researchers are working to study ability of countermeasures such as vaccines to prevent transmission in animal models.
SAVE is generating and characterizing reagents to be used across the USG and the global scientific community, including sera panels, viruses and protein. SAVE-generated and other available resources can be found at BEI Resource Repository.
SAVE has been involved in the research published in a variety of scientific journals focusing recently on the Omicron and Delta variants.
Federal agencies and laboratories, academic institutions, and corporations collaborate to make SAVE possible.
Office: 301-435-7244
Phone: 301-435-7244
Email: LymeDxStudies@niaid.nih.gov
Hours: Monday-Friday, 8:00 a.m. to 4:00 p.m.
A clinical trial has found that treatment with the immunomodulator interferon beta-1a plus the antiviral remdesivir was not superior to treatment with remdesivir alone in hospitalized adults with COVID-19 pneumonia. In addition, in a subgroup of patients who required high-flow oxygen, investigators found that interferon beta-1a was associated with more adverse events and worse outcomes. These findings were published today in the journal The Lancet Respiratory Medicine.
Submit a Media Request
Contact the NIAID News & Science Writing Branch.
This 10- to 20-minute survey is available for patients with eczema or parents of patients with eczema. These questions will help us learn more about patient experiences to improve current research and guide future research.
This online survey involves 10 to 20 minutes’ worth of questions.
Adults with eczema or parents/caregivers of a child with eczema.
This survey takes place online.
No
No
Office: Epithelial Therapeutics Unit
Phone: 301-451-8860
Email: Ashleigh.Sun@NIH.gov
The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, has awarded approximately $36.3 million to three academic institutions to conduct research to develop vaccines to protect against multiple types of coronaviruses and viral variants. The awards are intended to fuel vaccine research for a diverse family of coronaviruses, with a primary focus on potential pandemic-causing coronaviruses, such as SARS-CoV-2.
Submit a Media Request
Contact the NIAID News & Science Writing Branch.
NIAID established the Centers of Excellence for Influenza Research and Response (CEIRR) to study natural history, transmission, and pathogenesis of influenza and provide an international research infrastructure to address influenza outbreaks. CEIRR replaced the Centers of Excellence for Influenza Research and Surveillance (CEIRS) program, which was supported by contracts that concluded on March 31, 2021.
The CEIRR sites will conduct studies in the United States and internationally that follow cohorts of people to evaluate influenza-related research areas, such as understanding immune responses to vaccination and infection and identifying which immunological factors can determine influenza disease severity. They also will undertake projects on influenza surveillance, including transmission of influenza viruses from animals to humans (zoonotic transmission) to better understand how influenza viruses evolve, adapt, and transmit. The sites will prepare studies that could be rapidly launched as part of emergency research responses to outbreaks of influenza and other emerging viral pathogens.
Although CEIRR is primarily focused on influenza, the network also will study SARS-CoV-2, the virus that causes COVID-19, and other emerging viruses of pandemic potential.
NIAID also established the Influenza Data Processing and Communication Center (iDPCC) in parallel with the CEIRR contracts. The iDPCC will support the data processing, coordination, and reporting of the CEIRR sites, as well as serving as a central communication resource. This will include extensive outreach to the broad scientific community to share information on NIAID’s influenza research programs and help facilitate access to influenza reagents and resources.
The Ichan School of Medicine at Mount Sinai in New York (PI: Adolfo Garcia-Sastre, Ph.D.)
The University of Pennsylvania in Philadelphia (PI: Scott Hensley, Ph.D.)
St. Jude Children’s Research Hospital in Memphis, Tennessee (PI: Richard Webby, Ph.D. and Co-PI Stacey Schultz-Cherry, Ph.D.)
Emory University in Atlanta (PI: Walter Orenstein, M.D. and Co-PI Anice Lowen, Ph.D.)
University of Georgia Research Foundation, Inc., Athens (PI: Stephen Mark Tompkins, Ph.D. and Co-PI: Pejman Rohani, Ph.D)
Digital Infuzion, Gaithersburg, MD. (PI: Stephan Bour, Ph.D.)
Reagents generated by CEIRR research will be made available to the scientific community through the BEI Resources Repository.
Submit a Media Request
Contact the NIAID News & Science Writing Branch.