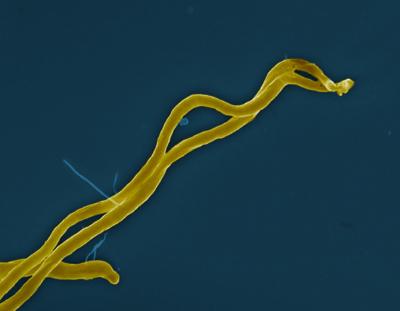
NIAID scientists and colleagues are investigating a potential treatment strategy against Lyme disease that would directly suppress Borrelia burgdorferi, the bacterium that causes the disease. If successful, their idea could reduce or end aggressive broad-spectrum antibiotic treatments that can be drawn-out and destroy the body’s helpful bacteria. The study appears in Frontiers in Antibiotics from scientists at NIAID’s Rocky Mountain Laboratories and colleagues at Purdue University.
The strategy involves the oligopeptide (Opp) transport system that most bacteria use as a secondary nutrition route to move small protein-like peptides through the bacteria. But B. burgdorferi, the researchers learned in a 2017 study, depends on the Opp system for survival, growth, and replication. They subsequently hypothesized that if they could impede the Opp system, maybe the bacterium would stop growing and die.
To test their theory they developed a method to screen 2,240 chemical compounds from a commercial library used for small-molecule drug discovery. They wanted to know if any compounds would bind to a prominent transport system protein known as OppA2. The research team identified eight compounds that did so, and of those, two compounds – C2 and C7 – significantly slowed B. burgdorferi growth, making the Opp system a viable, previously unexplored treatment target.
Next the scientists plan to screen more compounds, hoping to optimize binding to different OppA proteins (there are five to investigate) while still hindering B. burgdorferi growth.
“By targeting a system that appears to be only essential to Borrelia, it is possible that we could vastly improve current treatments by replacing them with a highly specific treatment that could reduce post-treatment complications,” study senior author Ashley Groshong, Ph.D., said.
Reference:
K Holly and A Kataria, et al. Unguarded Liabilities: Borrelia burgdorferi’s complex amino acid dependence exposes unique avenues of inhibition. Frontiers in Antibiotics DOI: 10.3389/frabi.2024.1395425 (2024).