Innate Immunity and Pathogenesis Section
Sonja M. Best, Ph.D.
Chief, Laboratory of Neurological Infections and Immunity

Major Areas of Research
- Mechanisms utilized by pathogenic viruses to modulate host innate immunity
- The role of novel IFN-stimulated genes (ISGs) in host resistance to virus infection
- The importance of dendritic cell (DC) function to anti-viral innate and adaptive immune responses
Program Description
The innate immune response is rapidly engaged after virus infection and functions to limit virus replication and mobilize adaptive immune responses. Viruses have evolved diverse strategies to suppress this critical host response and facilitate virus dissemination. In part, virus pathogenesis is a function of both the quality of the innate response evoked and the evasion strategies encoded by the invading pathogen. Thus, understanding the mechanisms underpinning immune activation and virus evasion will lead to improved vaccine design and novel therapeutics for treatment of both infectious and inflammatory disease.
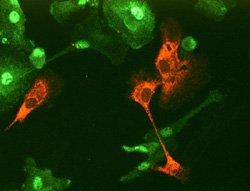
Figure 1: Human dendritic cells infected with LGTV and treated with type I IFN. The IFN-stimulated phosphorylation and nuclear localization of STAT1 (green) is inhibited in cells infected with LGTV (red).
Our current research program is shaped by the NIAID programmatic objectives in biodefense and emerging infectious disease research, namely basic research of pathogen biology and host response to develop effective vaccines and immunotherapeutics. We use flaviviruses (particularly members of the tick-borne encephalitis virus [TBEV] complex and West Nile virus [WNV]) as the primary model of infection to uncover novel cellular proteins requisite for antiviral innate immune responses and to identify virus-encoded mechanisms that antagonize these responses. These studies involve research of viruses at BSL2, BSL3 and BSL4. Our three main areas of research are outlined below.
- Mechanisms utilized by pathogenic viruses to modulate host innate immunity. Type I interferon (IFN) is essential to protection from flavivirus infection and has been used clinically as a potential therapeutic, albeit with limit success. This may be due to the observation that all flaviviruses examined to date antagonize IFN-dependent responses by suppressing JAK-STAT signal transduction. We originally identified NS5 as the major IFN antagonist encoded by flaviviruses, initially using Langat virus (LGTV; a member of the TBEV complex of flaviviruses) and later using WNV. Although other nonstructural proteins contribute to suppression of JAK-STAT signaling, studies by our laboratory and others suggest that NS5 is the most potent of the IFN antagonist proteins encoded by all vector-borne flaviviruses examined thus far. Hence, determining the mechanism(s) by which NS5 impedes signaling is essential to understand flavivirus pathogenesis and may lead to new therapeutic targets.
- The role of novel IFN-stimulated genes (ISGs) in host resistance to virus infection. It is important to understand the mechanisms underlying the anti-viral effects of IFN by identifying the function of ISGs with anti-viral activity. In particular, we are examining the role of tripartite motif (TRIM) proteins in host resistance to flavivirus replication. Many TRIM proteins are ISGs that mediate species-specific virus restriction or regulate host innate immunity. TRIM protein function is often dependent on their E3 Ubiquitin ligase activity. Therefore, we are also examining the role of ubiquitin in flavivirus replication and immune evasion.
- The importance of dendritic cell (DC) function to anti-viral innate and adaptive immune responses. It is essential to translate our findings to immunologically relevant cell types and animal models to understand the roles that induction and evasion of innate immunity has in development of adaptive immunity and in virus pathogenesis. We are using primary DCs and animal models to examine the importance of DCs as initial targets of infection, the role of DC pathogen recognition receptors in the immune response to infection, and how the mechanisms utilized by flaviviruses to modulate DC function affect adaptive immunity and virus pathogenesis.
Biography
Education
Ph.D., Australian National University
Dr. Best received her Ph.D. in biochemistry and molecular biology from the Australian National University where she studied the pathogenesis of myxoma virus, a poxvirus. She conducted her postdoctoral research at Rocky Mountain Laboratories (RML) on the complex role of apoptosis in the replication of parvoviruses. She stayed at RML as a Research Fellow and then a Staff Scientist to investigate virus-host interactions involved in flavivirus pathogenesis. It was during this time that she developed her interests in innate immunity and the molecular mechanisms utilized by flaviviruses to evade these critical host responses. In 2009, Dr. Best established an independent laboratory as a tenure-track investigator to expand her studies on interactions between pathogenic viruses and the host immune response. In 2011, Dr. Best was awarded a Presidential Early Career Award for Scientists and Engineers for her work on flavivirus suppression of innate immune responses.
Dr. Sonja Best, a virologist at NIAID's Rocky Mountain Laboratories, won a 2010 Presidential Early Career Award for Scientists and Engineers (PECASE), the highest honor bestowed by the U.S. government on scientists and engineers beginning their independent careers.
Selected Publications
Best SM. The many faces of the flavivirus NS5 proten in antagonism of type I interferon signaling. J Virol. 2017 Jan 18;9(3): e01970-16.
Dutta M, Robertson SJ, Okumura A, Scott DP, Chang J, Weiss JM, Sturdevant GL, Feldmann F, Haddock E, Chiramel AI, Ponia SS, Dougherty JD, Katze MG, Rasmussen AL, Best SM. A systems approach reveals MAVS signaling in myeloid cells as critical for resistance to ebola virus in murine models of infection. Cell Rep. 2017 Jan 17;18(3):816-829.
Grant A, Ponia SS, Tripathi S, Balasubramaniam V, Miorin L, Sourisseau M, Schwarz MC, Sánchez-Seco MP, Evans MJ, Best SM, García-Sastre A. Zika virus targets human STAT2 to inhibit type I interferon signaling. Cell Host Microbe. Cell Host Microbe. 2016 Jun 8;19(6):882-90.
Marzi A, Robertson SJ, Haddock E, Feldmann F, Hanley PW, Scott DP, Strong JE, Kobinger G, Best SM, Feldmann H. VSV-EBOV rabidly protects macaques against infection with the 2014/15 ebola virus outbreak strain. Science. 2015 Aug 14;349(6249):739-42.
Lubick KJ, Robertson SJ, McNally KL, Freedman BA, Rasmussen AL, Taylor RT, Walts AD, Tsuruda S, Sakai M, Ishizuka M, Boer EF, Foster EC, Chiramel AI, Addison CB, Green R, Kastner DL, Katze MG, Holland SM, Forlino A, Freeman AF, Boehm M, Yoshii K, Best SM. Flavivirus antagonism of type I interferon signaling reveals prolidase as a regulator of IFNAR1 surface expression. Cell Host Microbe. 2015 Jul 8;18(1):61-74.
Research Group
Shelly J. Robertson, Ph.D., Staff Scientist
Kristin McNally, Ph.D., Molecular Biologist
Gail Sturdevant, Biologist
Rebecca Broeckel, Ph.D., Postdoctoral Fellow
Abhilash Chiramel, Ph.D., Postdoctoral Fellow
Sanket Ponia, Ph.D., Postdoctoral Fellow
Emily Speranza, Ph.D., Postdoctoral Fellow

