Prion Cell Biology Unit
Established in 2017
Cathryn L. Haigh, Ph.D.
Chief, Prion Cell Biology Unit

Major Areas of Research
- Prion diseases
- Prion redox biology
- Prion protein processing and function
- Cerebral organoid models of prion infection and disease
Program Description
The Prion Cell Biology Unit is focused on elucidating the underlying cellular pathways causing neuronal death and dysfunction during prion diseases, as well as understanding the basic cellular functions of the prion protein. We are specifically interested in investigating: the role of the prion protein in cellular redox homeostasis; how perturbations in redox balance influence disease progression and toxicity; the influence of the prion protein on neural stem cell self-renewal and differentiation; and the functional outcomes of prion protein post-translational modifications.
The Unit is also dedicated to advancing the tools and techniques available for studying prion diseases. We have developed a human cerebral organoid model of prion infection that recapitulates certain features of human disease (see figure). The cerebral organoid model is being utilized to investigate the cellular and sub-cellular responses induced by infection with different sporadic Creutzfeldt-Jakob Disease (CJD) sub-types. We are additionally applying this technology to understanding the role of hereditary PRNP mutation in the causation of prion diseases.
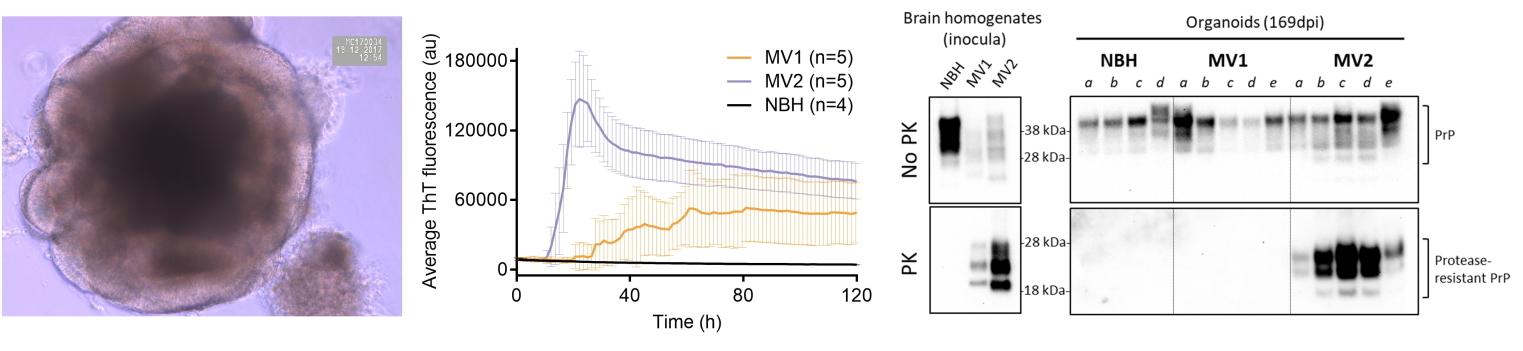
Figure shows a young (< 10 days old) human cerebral organoid forming organized regions of brain tissue (left). Middle; A prion seeding assay (real-time Quaking-Induced Conversion) of organoids that were infected with brain homogenates from one of two CJD subtypes, MV1 or MV2, and allowed to develop prion infection. Increased ThT fluorescence readings are indicative of increased prion seeds or infectious units. Normal brain homogenate (NBH) organoids are controls that remained un-infected. Right; Western blotting for protease-resistant prion protein (PrP), a biochemical indicator of the mis-folded prions that accumulate during human disease. In these examples differences are observed between the MV1 and MV2 CJD sub-type inoculums. Adapted from Groveman et al., 2019, Acta Neuropath Comms 7, 90.
Biography
Education
Ph.D., University of Bath (UK)
Dr. Haigh received her Ph.D. in Biochemistry from the University of Bath (UK). Her thesis focused on the cellular function of the prion protein and genetic control of its expression.
In July 2006, she relocated to The University of Melbourne (Australia) as a senior research officer in the Department of Pathology to continue her research into prion diseases. She subsequently held the positions of honorary research fellow at the Mental Health Research Institute of Victoria (Australia), senior research fellow in the Department of Medicine at the University of Melbourne (Australia), senior scientist for the Australian National CJD Registry, and additionally managed the prion containment facility within the Melbourne Brain Centre.
In 2017, Dr. Haigh was recruited to the National Institute of Allergy and Infectious Disease at the Rocky Mountain Laboratories in Hamilton (MT) and established the Prion Cell Biology Unit of which she is currently the chief.
Selected Publications
Foliaki ST, Groveman BR, Dews EA, Williams K, El Soufi H, Schwarz B, Leung JM, Schneider CA, Schwartz CL, Bohrnsen E, Kimzey CD, Race B, Haigh CL. Limbic system synaptic dysfunctions associated with prion disease onset. Acta Neuropathol Commun. 2024 Dec 20;12(1):192.
Groveman BR, Williams K, Race B, Foliaki S, Thomas T, Hughson AG, Walters RO, Zou W, Haigh CL. Lack of Transmission of Chronic Wasting Disease Prions to Human Cerebral Organoids. Emerg Infect Dis. 2024 Jun;30(6):1193-1202.
Williams K, Foliaki ST, Race B, Smith A, Thomas T, Groveman BR, Haigh CL. Neural cell engraftment therapy for sporadic Creutzfeldt-Jakob disease restores neuroelectrophysiological parameters in a cerebral organoid model. Stem Cell Res Ther. 2023 Dec 5;14(1):348.
Foliaki ST, Wood A, Williams K, Smith A, Walters RO, Baune C, Groveman BR, Haigh CL. Temporary alteration of neuronal network communication is a protective response to redox imbalance that requires GPI-anchored prion protein. Redox Biol. 2023 May 5;63:102733. doi: 10.1016/j.redox.2023.102733.
Groveman BR, Race B, Foliaki ST, Williams K, Hughson AG, Baune C, Zanusso G, Haigh CL. Sporadic Creutzfeldt-Jakob disease infected human cerebral organoids retain the original human brain subtype features following transmission to humanized transgenic mice. Acta Neuropathol Commun. 2023 Feb 14;11(1):28. doi: 10.1186/s40478-023-01512-1.
Foliaki ST, Smith A, Schwarz B, Bohrnsen E, Bosio CM, Williams K, Orrú CD, Lachenauer H, Groveman BR, Haigh CL. Altered energy metabolism in Fatal Familial Insomnia cerebral organoids is associated with astrogliosis and neuronal dysfunction. PLoS Genet. 2023 Jan 19;19(1):e1010565. doi: 10.1371/journal.pgen.1010565.
Research Group

From left to right: Simote Foliaki, , Hadil El Soufi, Arielle Hay, Katie Williams, Cathryn Haigh, Taylor Fletcher (alumni) and Bradley Groveman.

