TSE/Prion and Retroviral Pathogenesis Section
James A. Carroll, Ph.D.
Associate Scientist

Major Areas of Research
- Neuroinflammation during preclinical and clinical prion infection
- Influence of microglia and neurotoxic astrocytes on prion pathogenesis
- Alterations in cell populations and gene expression in the central nervous system and retina after prion infection
- Modeling Neuroborreliosis in human-derived neurons, astrocytes, and organoids
Program Description
Dr. Carroll’s primary research is concerned with understanding how neuroinflammation and glial cell activation (astrocytes and microglia) influence prion pathogenesis and neurodegeneration. Prions are infectious misfolded conformers of the cellular GPI-anchored protein PrP and can spread from cell to cell within the brain by seeded-polymerization. This group of proteinopathies can affect humans, cattle, sheep, and cervids and are resistant to many standard decontamination methods. Initially, it was assumed that prion diseases lack an immunological component due to the absence of a prominent antibody or interferon response. However, Dr. Carroll’s research has shown that prion infection elicits a substantial inflammatory response in the CNS and that many inflammatory effectors increase in expression in response to prion infection.
To address the potential impact of microglia in prion disease, Dr. Carroll performed several studies using the potent CSF-R1 inhibitor PLX5622 to reduce microglia in the CNS. These studies indicated that microglia were indispensable to host defense against prion disease. Moreover, his research implicated astrocytes as potentially affecting pathology during disease, where when microglia were absent, the astrocytes were more highly active, expressing numerous disease-related components. This has led to further investigations to assess astrogliosis during prion infection.

A Uniform Manifold Approximation and Projection (UMAP) of single nuclei RNA (snRNA) sequencing analysis of uninfected and prion-infected mouse thalamus depicting 43 transcriptional clusters from >69,000 nuclei examined.
Using high-throughput deep sequencing of RNA transcripts in longitudinal studies, Dr. Carroll has identified numerous differentially expressed genes in the CNS during prion infection. These investigations have yielded compelling results and suggest that microglia in the prion-infected brain assume an alternative phenotype that is distinct from those seen in other brain disorders. From these RNA-seq studies, it was determined that reactive astrocytes assume an expression signature that is not reliant on the canonical signals described in other neuroinflammatory models. Furthermore, this prion-specific reactive astrocyte expression signature is exacerbated when microglia are reduced in the CNS.
Dr. Carroll has begun to analyze the individual cellular changes in the brain using single nuclei RNA (snRNA) sequencing to better understand the relevant changes in the cell populations in the complex milieu of the CNS during infection. Thus far, the research has focused on gene expression changes in the thalamus at pre- and early-clinical times. The thalamus is affected early during prion infection in rodent models, and thalamic pathology is a key feature in human forms of prion disease.
A new aspect of Dr. Carroll’s research is a collaboration with Dr. Cathryn Haigh (Chief, Prion Cell Biology Unit, NIAID) to study Lyme Neuroborreliosis (LNB). Lyme disease, a global public health concern, is the most common tick-borne disease in North America and Eurasia, with an estimated 14% of the world’s population having become infected. Reported cases of Lyme disease in the U.S. have been on the rise for many years, with over 62,000 confirmed cases reported in 2022, making it the leading reportable arthropod-borne infectious disease. The Centers for Disease Control estimates that the disease is underreported, and the true incidence of Lyme disease in the U.S. is approaching 500,000 cases annually. Lyme disease, caused by bacterial spirochetes of the genus Borrelia, is a multi-systemic disorder affecting the skin, heart, central nervous system, and joints.
To address the need for additional models of LNB and to better understand the responses of the human CNS when exposed to Borrelia, this collaboration has developed two in vitro model systems. The first uses human cerebral organoids differentiated from human induced pluripotent stem cells (iPSCs) as an in vitro tissue model. The second uses iPSCs for differentiation into specific neuronal subtypes, astrocytes, and microglia-like cells for study. Exploiting these human-derived model systems, we are assessing responses to Borrelia infection that are stimulated in isolated cells from specific responses that only occur in these cells when they are part of an integrated organoid network. This project incorporates several cutting-edge technologies, including organoid development, bulk and single-cell RNA sequencing, metabolomics, and lipidomics.
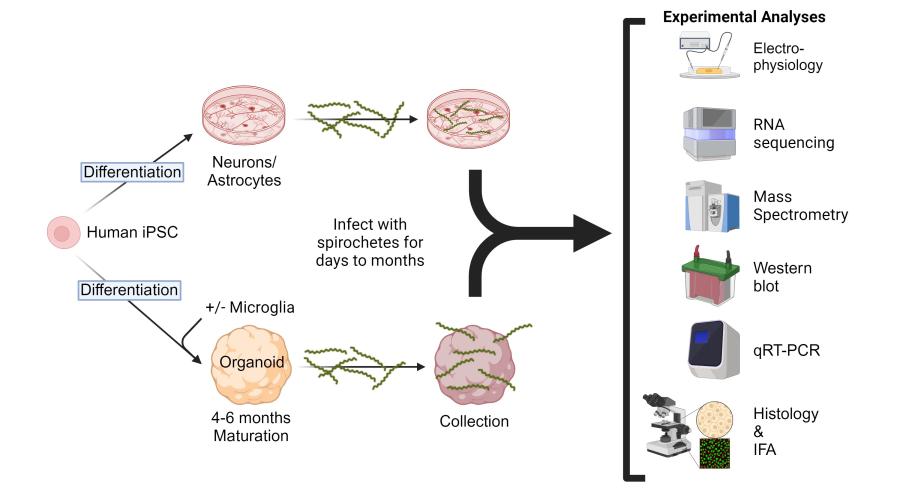
Experimental design and strategy to address potential responses of human cerebral organoids, astrocytes, and neurons after exposure to infectious Borrelia species that cause Lyme Neuroborreliosis.
Biography
Education
Ph.D., 1997, University of Georgia
Dr. James A. Carroll earned his Ph.D. in 1997 from the University of Georgia from the Department of Microbiology, studying virulence-associated membrane proteins expressed by Borrelia burgdorferi, the causative agent of Lyme disease. Following a postdoctoral fellowship at Rocky Mountain Laboratories (RML), NIAID, NIH investigating the environmental cues that influence gene regulation and virulence determinants in Borrelia burgdorferi, he joined the faculty of the Department of Microbiology and Molecular Genetics, University of Pittsburgh School of Medicine as an assistant professor in 2002. In late 2009, he was recruited back to RML to develop proteomics approaches in the search for additional biomarkers in prion diseases. Later, he branched out into transcriptomics to better assess the role of glia cells and neuroinflammation in the process of neurodegeneration that occurs in the late phase of prion infection. For his contributions to the Borrelia burgdorferi field, he received two awards from the Centers for Disease Control in 2011 (the James H. Nakano Citation and the Charles C. Shepard Science Award), and he was awarded the title of associate scientist in recognition of exceptional achievements in prion research as an investigator at the NIAID Division of Intramural Research.
Selected Publications
Carroll JA, Striebel JF, Baune C, Chesebro B, Race B. CD11c is not required by microglia to convey neuroprotection after prion infection. PLoS One. 2023 Nov 1;18(11):e0293301.
Carroll JA, Race B, Williams K, Striebel JF, Chesebro B. Innate immune responses after stimulation with Toll-like receptor agonists in ex vivo microglial cultures and an in vivo model using mice with reduced microglia. J Neuroinflammation. 2021 Sep 6;18(1):194.
Carroll JA, Foliaki ST, Haigh CL. A 3D cell culture approach for studying neuroinflammation. J Neurosci Methods. 2021 Jul 1;358:109201.
Carroll JA, Race B, Williams K, Striebel J, Chesebro B. RNA-seq and network analysis reveal unique glial gene expression signatures during prion infection. Mol Brain. 2020 May 7;13(1):71.
Carroll JA, Race B, Williams K, Striebel J, Chesebro B. Microglia Are Critical in Host Defense against Prion Disease. J Virol. 2018 Jul 17;92(15):e00549-18.
Carroll J.A., J.F. Striebel, A. Rangel, T. Woods, K. Phillips, K.E. Peterson, B. Race, and B. Chesebro. 2016. Prion strain differences in accumulation of PrPSc on neurons and glia are associated with similar expression profiles of neuroinflammatory genes: comparison of three prion strains. PLoS Path. Apr 5;12(4):e1005551.
Awards
- 1990 Graduated Cum Laude from Clemson University.
- 1993 and 1994 Recipient: Outstanding performance in research and teaching merit award from the Graduate School of the University of Georgia.
- 1997-2002 Recipient: Intramural Research Training Award (IRTA), NIAID, NIH.
- 2001 Recipient: NIAID Richard Asofsky Special Achievement Award in Equal Employment Opportunity in recognition of participation in the B.R.A.S.S. program.
- 2011 Recipient: the James H. Nakano Citation from the Centers for Disease Control for outstanding scientific article Gilmore et al. 2010. PNAS. 107(16):7515-7520.
- 2011 Recipient: the Charles C. Shepard Science Award, the highest CDC award for excellence in science, for an outstanding scientific article published in 2010 (Gilmore et al. PNAS. 107(16):7515-7520).
- 2019 Recipient: National Institutes of Health, NIAID 10 Years of Service award.
- 2021 Recipient: Honorific title of Associate Scientist in recognition of exceptional achievements as a Staff Scientist in the NIAID Division of Intramural Research.

