We apply systems immunology approaches to investigate human cutaneous leishmaniasis, a disfiguring chronic skin disease caused by the protozoan parasite Leishmania.
Cutaneous leishmaniasis (CL) is endemic and emerging in the southwestern United States and often also affects military personnel who have been deployed to Leishmania-endemic areas. Leishmania parasites are transmitted to humans via the bite of an infected sand fly. As the disease progresses, the immune response to the parasite in the skin can trigger pathologic inflammation that leads to chronic ulceration and permanent scarring. Current therapies for CL are variably effective with increasing reports of drug resistance. A major roadblock to the development of novel therapeutics for CL is our incomplete understanding of human immunity to Leishmania in the skin.
Our group seeks to define the cellular circuits that drive pathologic inflammation and damage to skin tissue in CL. To do this, we employ a variety of experimental and bioinformatic approaches, including single-cell transcriptomics, multiomic spatial profiling, and mechanistic multiscale tissue modeling to map the cell subsets and intercellular signals that drive CL immunopathology. Current efforts are focused on constructing a comprehensive single-cell and spatial atlas of human CL. This work is being performed in collaboration with the NIAID Leishmaniasis Clinic (Principal Investigator: Dr. Elise O’Connell, Laboratory of Parasitic Diseases). Our goal is to develop novel host-directed therapies for CL patients that 1) inhibit the critical signaling circuits driving pathologic inflammation in the skin and 2) enhance immune control of the parasite.
Another major focus of our group is investigating human immunity to vector bites and vector-borne pathogens via controlled human vector challenge studies. The immune response to vector bites profoundly affects the susceptibility of the host to vector-borne pathogens. In collaboration with Dr. Matthew Memoli (Laboratory of Infectious Diseases), we performed a human challenge study using mosquitos and sand flies, which revealed a conserved transcriptomic skin response against highly divergent vector species. We are now using single-cell and spatial profiling to map the cellular circuitry of the human skin response to vector bites. Our goal is to develop novel clinical therapies that co-opt these responses to enhance protection against vector-borne pathogens.
- Diagnosis and Treatment of Leishmania Infections: NCT00344188
- Human Immune Response to Ixodes Scapularis Tick Bites: NCT05036707
- Sample Collection From Healthy Volunteers for Assay Optimization: NCT03538600
- Obtaining Solid Tumor Tissue From People Having Biopsy or Surgery for Certain Types of Cancer: NCT01915225
Abdeladhim M, Teixeira C, Ressner R, Hummer K, Dey R, Gomes R, de Castro W, de Araujo FF, Turiansky GW, Iniguez E, Meneses C, Oliveira F, Aronson N, Lacsina JR*, Valenzuela JG*, Kamhawi S*. Lutzomyia longipalpis salivary proteins elicit human innate and adaptive immune responses detrimental to Leishmania parasites. bioRxiv. 2025 Mar 4; 2025.02.25.640210.
Lacsina JR, Kissinger R, Doehl JSP, Disotuar MM, Petrellis G, Short M, Lowe E, Oristian J, Sonenshine D, DeSouza-Vieira T. Host skin immunity to arthropod vector bites: from mice to humans. Frontiers in Tropical Diseases. 2024 May; 5:1308585.
Friedman-Klabanoff DJ, Birkhold M, Short MT, Wilson TR, Meneses CR, Lacsina JR, Oliveira F, Kamhawi S, Valenzuela JG, Hunsberger S, Mateja A, Stoloff G, Pleguezuelos O, Memoli MJ, Laurens MB. Safety and immunogenicity of AGS-v PLUS, a mosquito saliva peptide vaccine against arboviral diseases: A randomized, double-blind, placebo-controlled Phase 1 trial. EBioMedicine. 2022 Dec;86:104375.
DeSouza-Vieira T, Iniguez E, Serafim TD, de Castro W, Karmakar S, Disotuar MM, Cecilio P, Lacsina JR, Meneses C, Nagata BM, Cardoso S, Sonenshine DE, Moore IN, Borges VM, Dey R, Soares MP, Nakhasi HL, Oliveira F, Valenzuela JG, Kamhawi S. Heme Oxygenase-1 Induction by Blood-Feeding Arthropods Controls Skin Inflammation and Promotes Disease Tolerance. Cell Rep. 2020 Oct 27;33(4):108317.
Lacsina JR, Marks OA, Liu X, Reid DW, Jagannathan S, Nicchitta CV. Premature translational termination products are rapidly degraded substrates for MHC class I presentation. PLoS One. 2012;7(12):e51968.
Lacsina JR, LaMonte G, Nicchitta CV, Chi JT. Polysome profiling of the malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 2011 Sep;179(1):42-6.
- Human immunity to Leishmania parasites and to arthropod vector bites (ticks, mosquitos, and sand flies)
- Systems immunology of human skin inflammation: single-cell RNA-seq and multiomic spatial profiling
- Controlled human challenge studies of vector bites and vector-transmitted pathogens
- Mechanistic multiscale tissue modeling of skin inflammation
Notice of Special Interest (NOSI)—Research on the Health of Bisexual and Bisexual+ People
James A. Carroll, Ph.D.
Dr. Carroll’s primary research is concerned with understanding how neuroinflammation and glial cell activation (astrocytes and microglia) influence prion pathogenesis and neurodegeneration. Prions are infectious misfolded conformers of the cellular GPI-anchored protein PrP and can spread from cell to cell within the brain by seeded-polymerization. This group of proteinopathies can affect humans, cattle, sheep, and cervids and are resistant to many standard decontamination methods. Initially, it was assumed that prion diseases lack an immunological component due to the absence of a prominent antibody or interferon response. However, Dr. Carroll’s research has shown that prion infection elicits a substantial inflammatory response in the CNS and that many inflammatory effectors increase in expression in response to prion infection.
To address the potential impact of microglia in prion disease, Dr. Carroll performed several studies using the potent CSF-R1 inhibitor PLX5622 to reduce microglia in the CNS. These studies indicated that microglia were indispensable to host defense against prion disease. Moreover, his research implicated astrocytes as potentially affecting pathology during disease, where when microglia were absent, the astrocytes were more highly active, expressing numerous disease-related components. This has led to further investigations to assess astrogliosis during prion infection.

A Uniform Manifold Approximation and Projection (UMAP) of single nuclei RNA (snRNA) sequencing analysis of uninfected and prion-infected mouse thalamus depicting 43 transcriptional clusters from >69,000 nuclei examined.
Using high-throughput deep sequencing of RNA transcripts in longitudinal studies, Dr. Carroll has identified numerous differentially expressed genes in the CNS during prion infection. These investigations have yielded compelling results and suggest that microglia in the prion-infected brain assume an alternative phenotype that is distinct from those seen in other brain disorders. From these RNA-seq studies, it was determined that reactive astrocytes assume an expression signature that is not reliant on the canonical signals described in other neuroinflammatory models. Furthermore, this prion-specific reactive astrocyte expression signature is exacerbated when microglia are reduced in the CNS.
Dr. Carroll has begun to analyze the individual cellular changes in the brain using single nuclei RNA (snRNA) sequencing to better understand the relevant changes in the cell populations in the complex milieu of the CNS during infection. Thus far, the research has focused on gene expression changes in the thalamus at pre- and early-clinical times. The thalamus is affected early during prion infection in rodent models, and thalamic pathology is a key feature in human forms of prion disease.
A new aspect of Dr. Carroll’s research is a collaboration with Dr. Cathryn Haigh (Chief, Prion Cell Biology Unit, NIAID) to study Lyme Neuroborreliosis (LNB). Lyme disease, a global public health concern, is the most common tick-borne disease in North America and Eurasia, with an estimated 14% of the world’s population having become infected. Reported cases of Lyme disease in the U.S. have been on the rise for many years, with over 62,000 confirmed cases reported in 2022, making it the leading reportable arthropod-borne infectious disease. The Centers for Disease Control estimates that the disease is underreported, and the true incidence of Lyme disease in the U.S. is approaching 500,000 cases annually. Lyme disease, caused by bacterial spirochetes of the genus Borrelia, is a multi-systemic disorder affecting the skin, heart, central nervous system, and joints.
To address the need for additional models of LNB and to better understand the responses of the human CNS when exposed to Borrelia, this collaboration has developed two in vitro model systems. The first uses human cerebral organoids differentiated from human induced pluripotent stem cells (iPSCs) as an in vitro tissue model. The second uses iPSCs for differentiation into specific neuronal subtypes, astrocytes, and microglia-like cells for study. Exploiting these human-derived model systems, we are assessing responses to Borrelia infection that are stimulated in isolated cells from specific responses that only occur in these cells when they are part of an integrated organoid network. This project incorporates several cutting-edge technologies, including organoid development, bulk and single-cell RNA sequencing, metabolomics, and lipidomics.
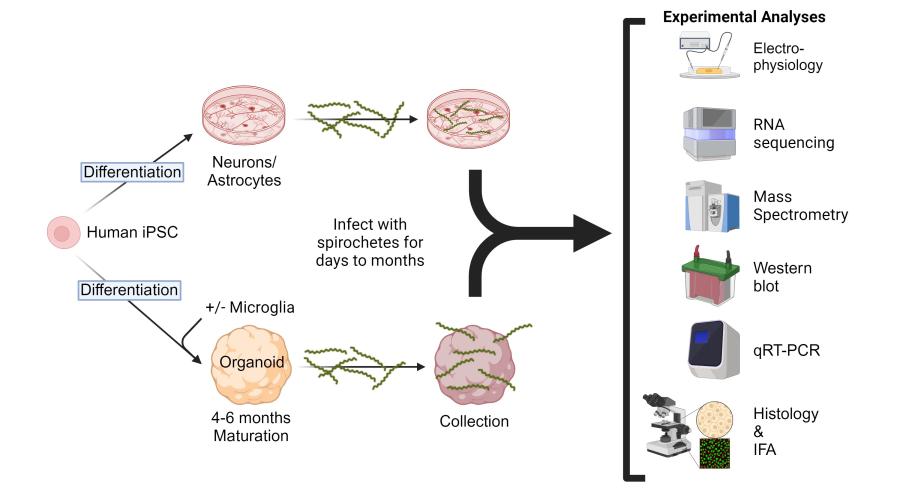
Experimental design and strategy to address potential responses of human cerebral organoids, astrocytes, and neurons after exposure to infectious Borrelia species that cause Lyme Neuroborreliosis.
Carroll JA, Striebel JF, Baune C, Chesebro B, Race B. CD11c is not required by microglia to convey neuroprotection after prion infection. PLoS One. 2023 Nov 1;18(11):e0293301.
Carroll JA, Race B, Williams K, Striebel JF, Chesebro B. Innate immune responses after stimulation with Toll-like receptor agonists in ex vivo microglial cultures and an in vivo model using mice with reduced microglia. J Neuroinflammation. 2021 Sep 6;18(1):194.
Carroll JA, Foliaki ST, Haigh CL. A 3D cell culture approach for studying neuroinflammation. J Neurosci Methods. 2021 Jul 1;358:109201.
Carroll JA, Race B, Williams K, Striebel J, Chesebro B. RNA-seq and network analysis reveal unique glial gene expression signatures during prion infection. Mol Brain. 2020 May 7;13(1):71.
Carroll JA, Race B, Williams K, Striebel J, Chesebro B. Microglia Are Critical in Host Defense against Prion Disease. J Virol. 2018 Jul 17;92(15):e00549-18.
Carroll J.A., J.F. Striebel, A. Rangel, T. Woods, K. Phillips, K.E. Peterson, B. Race, and B. Chesebro. 2016. Prion strain differences in accumulation of PrPSc on neurons and glia are associated with similar expression profiles of neuroinflammatory genes: comparison of three prion strains. PLoS Path. Apr 5;12(4):e1005551.
Awards
- 1990 Graduated Cum Laude from Clemson University.
- 1993 and 1994 Recipient: Outstanding performance in research and teaching merit award from the Graduate School of the University of Georgia.
- 1997-2002 Recipient: Intramural Research Training Award (IRTA), NIAID, NIH.
- 2001 Recipient: NIAID Richard Asofsky Special Achievement Award in Equal Employment Opportunity in recognition of participation in the B.R.A.S.S. program.
- 2011 Recipient: the James H. Nakano Citation from the Centers for Disease Control for outstanding scientific article Gilmore et al. 2010. PNAS. 107(16):7515-7520.
- 2011 Recipient: the Charles C. Shepard Science Award, the highest CDC award for excellence in science, for an outstanding scientific article published in 2010 (Gilmore et al. PNAS. 107(16):7515-7520).
- 2019 Recipient: National Institutes of Health, NIAID 10 Years of Service award.
- 2021 Recipient: Honorific title of Associate Scientist in recognition of exceptional achievements as a Staff Scientist in the NIAID Division of Intramural Research.
- Neuroinflammation during preclinical and clinical prion infection
- Influence of microglia and neurotoxic astrocytes on prion pathogenesis
- Alterations in cell populations and gene expression in the central nervous system and retina after prion infection
- Modeling Neuroborreliosis in human-derived neurons, astrocytes, and organoids
Joseph P. Casazza, M.D., Ph.D.
My work at the NIH concentrates on two aspects of HIV infection: the control of HIV-infection by the immunologic mechanism and the description of the changes in the CD4 T cell transcriptome caused by HIV-infection. In collaboration with individuals at the VRC, the California Institute of Technology and the Ragon Institute I am responsible for a clinical trial in which an AAV vector carries the coding sequence for VRC07, a potent broadly neutralizing Ab, into muscle cells of HIV-infected individuals on effective anti-retroviral therapy. In some individuals production of VRC07 occurred at ug/ml serum quantities for over 3 years. Although this level of VRC07 is not protective, this study shows that it is possible to side-step some of the difficulties in producing an immunogen capable of inducing a broadly neutralizing antibody by using a viral vector to transduction muscle cells. I have also established methods to identify and sort live HIV-infected CD4 T cells. Unlike matrix proteins, envelope proteins are fully mature when transported to the surface of CD4 T cells. By using fluorescently labeled broadly neutralizing antibodies that bind HIV envelope protein expressed on the surface of CD4 T cells, it is possible to use index sorting to identify live HIV-infected CD4 T cells. The transcriptomes of these cells are then characterized using RNA seq. We have used these methods to characterize the transcriptomes from HIV-infected peripheral CD4 T cells and in ACH2 cells transitioning from “latent-infection” to “active-infection”. These studies have allowed us to correlate markers of disease progression such as CD4 down regulation, viral RNA concentrations and viral RNA splice patterns, with activation of NF-B pathway and increased HIV-RNA transcription. We are currently using these methods to identify and characterize the longitudinal effect of SHIV infection on the CD4 T cell transcriptome of individual SHIV infected CD4 T cells from rhesus macaque lymph nodes.
VRC 200 (03-I-0263): Apheresis and Specimen Collection Procedures to Obtain Plasma, Peripheral Blood Mononuclear Cells (PBMCs) and Other Specimens for Research Studies- Associate Investigator.
VRC 323 (NIH 20-I-0145): A Phase I Open-Label Clinical Trial to Evaluate the Dose, Safety, Toerablity and Immunogenicity of an Influenza H10 Stabilized Stem Ferritin Vaccine, VRC-FLUNPF0103-VP, in Healthy Adults- Principal Investigator
VRC 325 (NIH000410): A Phase I Open-Label Clinical Trial to Evaluate the Dose, Safety, Tolerability and Immunogenicity of Mosaic Quadrivalent Influenza Vaccine Compared with a Licensed Inactiviated Seasona QIV, in Healthy Adults – Associate Investigator
VRC-603 (NIH-18-I-0030): A Phase 1 Dose-Escalation Study of the Safety of AAV8-VRC07 (VRC-HIVAAV070-00-GT) Recombinant AAV Vector Expressing VRC07 HIV-1 Neutralizing Antibody in Antiretroviral-Treated, HIV-1 Infected Adults with Controlled Viremia -Principal Investigator.
VRC609 (NIH 20-I-0096) A Phase I Open-Label Dose Escalation Study of the Safety and Pharmacokinetics of a Human Monoclonal Antibody, VRC-HIVMAB091-00-AB (N6LS), Administered Intravenously or Subcutaneously to Healthy Adults- Medical Officer
VRC611 (NIH 000536) A Phase I Safety and Pharmacokinetics Study to Evaluate a Human Monoclonal Antibody (mAb) VRC-HIVMAB0102-00-AB (CAP256V2LS) Administered Via Subcutaneous and Intravenous Injection in Healthy Adults- Medical Officer
VRC 614 (NIH 000536) A Phase 1, Dose Escalation, Open-Label Clinical Trial with Experimental Controlled Human Malaria Infections (CHMI) to Evaluate Safety and Protective Efficacy of an Anti-Malaria Human Monoclonal Antibody, VRC-MALMAB0114-00-AB (L9LS), in Healthy, Malaria-Naive Adult- Medical Officer
VRC 900 (10-I-0109): Evaluation of Tissue-Specific Immune Responses in Adults 18 Years of Age and Older -Associate Investigator.
Casazza JP, Cale EM, Narpala S, Yamshchikov GV, Coates EE, Hendel CS, Novik L, Widge AT, Apte P, Gordon I, Gaudinski MR, Conan-Cibotti M, Lin BC, Trofymenko O, Telscher S, Plummer SA, Wycuff D, Adams WC, Pandey JP, McDermott A, Roederer M, Sukienik AN, Doria-Rose NA, O’Dell S, Gall JG, Flach B, Nason MC, Saunders KO, Stein JA, Schwartz RM, Balazs AB, Baltimore D, Nabel GJ, Koup RA, Graham BS, Ledgerwood JE, Mascola JR and the VRC 603 Study Team (2022) Nat Med. 2022 May;28(5):1022-1030. doi: 10.1038/s41591-022-01762-x. Epub 2022 Apr 11.PMID: 35411076.
Pegu A, Xu L, DeMouth ME, Fabozzi G, March K, Almasri CG, Cully MD, Wang K, Yang ES, Dias J, Fennessey CM, Hataye J, Wei RR, Rao E, Casazza JP, Promsote W, Asokan M, McKee K, Schmidt SD, Chen X, Liu C, Shi W, Geng H, Foulds KE, Kao SF, Noe A, Li H, Shaw GM, Zhou T, Petrovas C, Todd JP, Keele BF, Lifson JD, Doria-Rose NA, Koup RA, Yang ZY, Nabel GJ, Mascola JR. Potent anti-viral activity of a trispecific HIV neutralizing antibody in SHIV-infected monkeys. Cell Rep. 2022 Jan 4;38(1):110199.
Hataye JM, Casazza JP, Best K, Liang CJ, Immonen TT, Ambrozak DR, Darko S, Henry AR, Laboune F, Maldarelli F, Douek DC, Hengartner NW, Yamamoto T, Keele BF, Perelson AS, Koup RA. Principles Governing Establishment versus Collapse of HIV-1 Cellular Spread. Cell Host Microbe. 2019 Dec 11;26(6):748-763.e20.
Casazza JP, Bowman KA, Adzaku S, Smith EC, Enama ME, Bailer RT, Price DA, Gostick E, Gordon IJ, Ambrozak DR, Nason MC, Roederer M, Andrews CA, Maldarelli FM, Wiegand A, Kearney MF, Persaud D, Ziemniak C, Gottardo R, Ledgerwood JE, Graham BS, Koup RA; VRC 101 Study Team. Therapeutic vaccination expands and improves the function of the HIV-specific memory T-cell repertoire. J Infect Dis. 2013 Jun 15;207(12):1829-40.
Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J Exp Med. 2006 Dec 25;203(13):2865-77.
- HIV Vaccines
- HIV pathogenesis
Howard E. Boudreau, Ph.D.
We are currently investigating the role of mutant p53-induced NOX4 on the cancer cell secretome, and the effects NOX4-derived reactive oxygen species have on the inflammatory tumor microenvironment.
Ma WF, Boudreau HE, Leto TL. Pan-Cancer Analysis Shows TP53 Mutations Modulate the Association of NOX4 with Genetic Programs of Cancer Progression and Clinical Outcome. Antioxidants (Basel). 2021 Feb 4;10(2):235.
Boudreau HE, Leto TL. Model Systems to Investigate NOX-Dependent Cell Migration and Invasiveness. Methods Mol Biol. 2019;1982:473-485.
Sugamata R, Donko A, Murakami Y, Boudreau HE, Qi CF, Kwon J, Leto TL. Duox1 Regulates Primary B Cell Function under the Influence of IL-4 through BCR-Mediated Generation of Hydrogen Peroxide. J Immunol. 2019 Jan 15;202(2):428-440.
Boudreau HE, Ma WF, Korzeniowska A, Park JJ, Bhagwat MA, Leto TL. Histone modifications affect differential regulation of TGFβ- induced NADPH oxidase 4 (NOX4) by wild-type and mutant p53. Oncotarget. 2017 Jul 4;8(27):44379-44397.
Boudreau HE, Casterline BW, Burke DJ, Leto TL. Wild-type and mutant p53 differentially regulate NADPH oxidase 4 in TGF-β-mediated migration of human lung and breast epithelial cells. Br J Cancer. 2014 May 13;110(10):2569-82.
- NADPH oxidase (NOX) enzyme function
- Inflammation
- Wound healing
- Cancer metastasis
Suk See De Ravin, M.D., Ph.D.
Dr. De Ravin’s primary goal is to develop novel gene therapy and cell therapy approaches for treatment of Inborn Errors of Immunity (IEI) /Primary Immunodeficiency Diseases (PID). Advances in genomic diagnosis and immune-phenotype characterization within National Institutes of Allergy and Infectious Diseases identifies many individuals who will benefit from gene and cell therapy. Current gene therapy for IEI (e.g., X-linked SCID, Chronic Granulomatous Disease (CGD) using lentivectors) has provided clinical benefit to multiple patients. However, the risk semi-random vector insertion causing insertional oncogenesis and the lack of physiological gene expression from inserted exogenous transgenes leave room for improvement. To address these concerns, Dr. De Ravin is working on targeted approaches to insert therapeutic genes in hematopoietic stem and progenitor cells for future gene therapy. Programmable CRISPR-Cas9 nuclease systems can deliver corrective genes or repair mutations efficiently. However, this approach carries risks of genotoxicity although there are mitigating agents. Base editing that side-steps risks associated with double strand DNA breaks and the dependence on homology-directed repair is another promising approach for gene therapy for the near future. For patients with infections difficult to control with current antimicrobials, mRNA transfection of autologous primary cells such as granulocytes provide hopes for a transient therapeutic approach. A better understanding of the immune-phenotype allows rational designs for short-term disease control and ultimately long-term treatment of disease with ideal gene therapy approach.
NADPH Oxidase Correction in mRNA-transfected Granulocyte-enriched Cells in Chronic Granulomatous Disease (CGD): NCT05189925
Recruitment and Apheresis Collection of Peripheral Blood Hematopoietic Stem Cells, Mononuclear Cells and Granulocytes: NCT00001405
Lentiviral Gene Transfer for Treatment of Children Older Than Two Years of Age With X-Linked Severe Combined Immunodeficiency (XSCID): NCT01306019
Screening and Baseline Assessment of Patients with Abnormalities of Immune Function
A Phase 1 Study to Evaluate the Safety and Tolerability of Tandemly-purified Allogeneic CD34+CD90+ HSC Administered Following Conditioning with JAS 191 to Achieve Engraftment and Immune Reconstitution in Patients with SCID
Brault J, Liu T, Bello E, Liu S, Sweeney CL, Meis RJ, Koontz S, Corsino C, Choi U, Vayssiere G, Bosticardo M, Dowdell K, Lazzarotto CR, Clark AB, Notarangelo LD, Ravell JC, Lenardo MJ, Kleinstiver BP, Tsai SQ, Wu X, Dahl GA, Malech HL, De Ravin SS. CRISPR-targeted MAGT1 insertion restores XMEN patient hematopoietic stem cells and lymphocytes. Blood. 2021 Dec 30;138(26):2768-2780.
De Ravin SS, Brault J, Meis RJ, Liu S, Li L, Pavel-Dinu M, Lazzarotto CR, Liu T, Koontz SM, Choi U, Sweeney CL, Theobald N, Lee G, Clark AB, Burkett SS, Kleinstiver BP, Porteus MH, Tsai S, Kuhns DB, Dahl GA, Headey S, Wu X, Malech HL. Enhanced homology-directed repair for highly efficient gene editing in hematopoietic stem/progenitor cells. Blood. 2021 May 13;137(19):2598-2608.
Brault J, Meis RJ, Li L, Bello E, Liu T, Sweeney CL, Koontz SM, Dowdell K, Theobald N, Lee J, Allen C, Clark AB, Ravell JC, Lenardo MJ, Dahl GA, Malech HL, De Ravin SS. MAGT1 messenger RNA-corrected autologous T and natural killer cells for potential cell therapy in X-linked immunodeficiency with magnesium defect, Epstein-Barr virus infection and neoplasia disease. Cytotherapy. 2021 Mar;23(3):203-210.
De Ravin SS, Brault J, Meis RJ, Li L, Theobald N, Bonifacino AC, Lei H, Liu TQ, Koontz S, Corsino C, Zarakas MA, Desai JV, Clark AB, Choi U, Metzger ME, West K, Highfill SL, Kang E, Kuhns DB, Lionakis MS, Stroncek DF, Dunbar CE, Tisdale JF, Donahue RE, Dahl GA, Malech HL. NADPH oxidase correction by mRNA transfection of apheresis granulocytes in chronic granulomatous disease. Blood Adv. 2020 Dec 8;4(23):5976-5987.
De Ravin SS, Wu X, Moir S, Anaya-O'Brien S, Kwatemaa N, Littel P, Theobald N, Choi U, Su L, Marquesen M, Hilligoss D, Lee J, Buckner CM, Zarember KA, O'Connor G, McVicar D, Kuhns D, Throm RE, Zhou S, Notarangelo LD, Hanson IC, Cowan MJ, Kang E, Hadigan C, Meagher M, Gray JT, Sorrentino BP, Malech HL, Kardava L. Lentiviral hematopoietic stem cell gene therapy for X-linked severe combined immunodeficiency. Sci Transl Med. 2016 Apr 20;8(335):335ra57.
De Ravin SS, Reik A, Liu PQ, Li L, Wu X, Su L, Raley C, Theobald N, Choi U, Song AH, Chan A, Pearl JR, Paschon DE, Lee J, Newcombe H, Koontz S, Sweeney C, Shivak DA, Zarember KA, Peshwa MV, Gregory PD, Urnov FD, Malech HL. Targeted gene addition in human CD34(+) hematopoietic cells for correction of X-linked chronic granulomatous disease. Nat Biotechnol. 2016 Apr;34(4):424-9.
- Gene therapy
- Inborn Errors of Immunity/Primary Immunodeficiency Diseases; Chronic Granulomatous Disease, X-linked Severe Combined Immunodeficiency, XMEN, WHIM, XLA, STAT3, STAT1, CTLA4.
Farinaz Safavi, M.D., Ph.D.
Inborn Errors of Immunity (IEIs) are genetic disorders of the immune system with clinical manifestations of infection and autoinflammatory syndrome. Neurological disorders are one of the common causes of irreversible morbidity and mortality in patients with IEIs. Neuroinflammatory, neuroinfectious and neurodegenerative diseases have been reported extensively in this patient population but the role of immune related gene defects on development, function and immunoregulation of nervous system is still unknown.
The NeuroImmunopathogenesis Unit performs an integrated bench to bedside research to better understand the role of immunodeficiencies in nervous system. Taking a comprehensive approach to evaluate profile and function of immune cells in both blood and cerebrospinal fluid, the most adjacent cells to nervous system, provides valuable understanding about dynamic of immune cells and responses in CNS immune-compartment paving the path to find more targeted therapeutics. By using induced pluripotent stem cell technology, the unit also investigates the role of immune related gene defects in development and function of human neurons and glia to find underlying cellular and molecular pathways in immunodeficient patients with neurological disorders.
Furthermore, rare neuroinfectious, neuroinflammatory and neurodegenerative diseases with atypical clinical features can be a manifestation of immune related gene defects. Our holistic clinical and basic immunology, neuroscience and genetic approach facilitates to better understand the underlying mechanisms of these presentations to clarify diagnosis and treatments of this patients’ complex and often refractory to treatment neurological diseases.
Lee MH, Perl DP, Steiner J, Pasternack N, Li W, Maric D, Safavi F, Horkayne-Szakaly I, Jones R, Stram MN, Moncur JT, Hefti M, Folkerth RD, Nath A. Neurovascular injury with complement activation and inflammation in COVID-19. Brain. 2022 Jul 5:awac151.
Safavi F, Thome R, Li Z, Wang L, Rasouli J, Ciric B, Zhang GX, Rostami A. A serine protease inhibitor induces type 1 regulatory T cells through IFN-γ/STAT1 signaling. Cell Mol Immunol. 2020 Sep;17(9):1004-1006.
Safavi F, Nath A. Silencing of immune activation with methotrexate in patients with COVID-19. J Neurol Sci. 2020 Aug 15;415:116942.
Safavi F, Thome R, Li Z, Zhang GX, Rostami A. Dimethyl fumarate suppresses granulocyte macrophage colony-stimulating factor-producing Th1 cells in CNS neuroinflammation. Neurol Neuroimmunol Neuroinflamm. 2020 May 5;7(4):e729.
Rasouli J, Ciric B, Imitola J, Gonnella P, Hwang D, Mahajan K, Mari ER, Safavi F, Leist TP, Zhang GX, Rostami A. Expression of GM-CSF in T Cells Is Increased in Multiple Sclerosis and Suppressed by IFN-β Therapy. J Immunol. 2015 Jun 1;194(11):5085-93.
El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011 Jun;12(6):568-75.
- Neurological manifestations of primary and acquired immunodeficiency
- Role of immune related gene defects in neurons and glial cell function
- The effect of host immune defects on CNS immune-compartment
- Investigate the role of Inborn Errors of Immunity (IEIs) in patients with atypical neuroinflammatory, neuroinfectious and neurodegenerative diseases
Kalpana Manthiram, M.D.
The overall goal of Dr. Manthiram’s research is to elucidate genetic susceptibility factors and immunologic mechanisms of mucosal autoinflammatory disorders through translational research.
Her research group studies periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) syndrome, which is the most common periodic fever syndrome in children. Patients with PFAPA have recurrent, regular episodes of fever with aphthous ulcers pharyngitis, and cervical adenitis. She has focused on unraveling the pathogenesis of PFAPA syndrome by identifying genetic risk factors and studying tonsil immunology. She has identified common genetic susceptibility loci and class I and class II HLA risk alleles for PFAPA indicating the PFAPA is a complex genetic disease. These associated risk variants are also risk alleles for recurrent aphthous ulcers and Behçet’s disease, which links these three oropharyngeal disorders on a spectrum of disease called Behçet’s spectrum disorders. Recently, her group has studied the immune response to SARS-CoV-2 in the tonsil and adenoid tissue, shedding light on the mucosal immune response to this virus.
Her lab also studies the mechanism of other mucosal inflammatory disorders including obstructive sleep apnea and trisomy 8-associated autoinflammatory disease (TRIAD). Dr. Manthiram follows patients with PFAPA and TRIAD at NIH.
Manthiram K, Preite S, Dedeoglu F, Demir S, Ozen S, Edwards KM, Lapidus S, Katz AE; Genomic Ascertainment Cohort, Feder HM Jr, Lawton M, Licameli GR, Wright PF, Le J, Barron KS, Ombrello AK, Barham B, Romeo T, Jones A, Srinivasalu H, Mudd PA, DeBiasi RL, Gül A, Marshall GS, Jones OY, Chandrasekharappa SC, Stepanovskiy Y, Ferguson PJ, Schwartzberg PL, Remmers EF, Kastner DL. Common genetic susceptibility loci link PFAPA syndrome, Behçet's disease, and recurrent aphthous stomatitis. Proc Natl Acad Sci U S A. 2020 Jun 23;117(25):14405-14411.
Amarilyo G, Rothman D, Manthiram K, Edwards KM, Li SC, Marshall GS, Yildirim-Toruner C, Haines K, Ferguson PJ, Lionetti G, Cherian J, Zhao Y, DeLaMora P, Syverson G, Nativ S, Twilt M, Michelow IC, Stepanovskiy Y, Thatayatikom A, Harel L, Akoghlanian S, Tucker L, Marques MC, Srinivasalu H, Propst EJ, Licameli GR, Dedeoglu F, Lapidus S; CARRA PFAPA Consensus Treatment Plan Workgroup. Consensus treatment plans for periodic fever, aphthous stomatitis, pharyngitis and adenitis syndrome (PFAPA): a framework to evaluate treatment responses from the childhood arthritis and rheumatology research alliance (CARRA) PFAPA work group. Pediatr Rheumatol Online J. 2020 Apr 15;18(1):31.
Manthiram K, Correa H, Boyd K, Roland J, Edwards K. Unique histologic features of tonsils from patients with periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome. Clin Rheumatol. 2018 May;37(5):1309-1317.
Manthiram K, Nesbitt E, Morgan T, Edwards KM. Family History in Periodic Fever, Aphthous Stomatitis, Pharyngitis, Adenitis (PFAPA) Syndrome. Pediatrics. 2016 Sep;138(3):e20154572.
Videos
Treatment of Patients with PFAPA
Demystifying Medicine: Reimagining the Taxonomy of Autoinflammatory Disease
- Genetics of periodic fever, aphthous stomatitis, pharyngitis, adenitis (PFAPA) syndrome and other Behçet’s Spectrum Disorders
- Immunology of pediatric tonsil disorders
- Immune responses to infections in oropharyngeal lymphoid tissue
- Clinical and immunologic features of trisomy 8 associated autoinflammatory disease (TRIAD)
James M. Cherry, Ph.D.
The Research Technologies Branch (RTB) provides cutting edge expertise and research technologies primarily in support of NIAID intramural investigators. Our Lab was established to provide researchers access to leading-edge technologies and specialized expertise through a tightly integrated, highly effective approach to study complex biological problems. During the past 30 years, the advent of the biotechnology industry and the development of new scientific disciplines have resulted in an explosion of new technologies. In addition, advances in optics, lasers, and computational biology have revolutionized well-established disciplines such as microscopy (light and electron), flow cytometry, genomic and proteomics. These technologies require very expensive instrumentation platform integration and more importantly highly trained specialized scientists to adapt these new technologies to the research needs of the Institute’s diverse research agenda. The Branch implements state of the art research technologies and project-specific applications for the NIAID intramural research program. This is accomplished through collaboration with current researchers along with a network of facilities located in Bethesda, Rockville and Frederick, Maryland, as well as Hamilton, Montana. Scientists in the RTB make significant contributions to collaborative research projects with NIAID researchers and their extramural collaborators. Equally important is their dedication technology; a substantial portion of their efforts focuses on technology development, resulting in advances methods and approaches designed.
The Center for Human Immunology (CHI) mission is to achieve an integrated and predictive understanding of human immunity and immune-microbiome behavior and function in health and disease. This can be accomplished by large scale human studies, scientific expertise and a strong influx of technology applications that is not readily available in a single laboratory.
Pinto LA, Shawar RM, O'Leary B, Kemp TJ, Cherry J, Thornburg N, Miller CN, Gallagher PS, Stenzel T, Schuck B, Owen SM, Kondratovich M, Satheshkumar PS, Schuh A, Lester S, Cassetti MC, Sharpless NE, Gitterman S, Lowy DR. A Trans-Governmental Collaboration to Independently Evaluate SARS-CoV-2 Serology Assays. Microbiol Spectr. 2022 Feb 23;10(1):e0156421.
Rozenblum E, Sotelo-Silveira JR, Kim GY, Zhu JY, Lau CC, McNeil N, Korolevich S, Liao H, Cherry JM, Munroe DJ, Ried T, Meltzer PS, Kuehl WM, Roschke AV. Novel near-diploid ovarian cancer cell line derived from a highly aneuploid metastatic ovarian tumor. PLoS One. 2017 Aug 7;12(8):e0182610.
Cabarcas SM, Thomas S, Zhang X, Cherry JM, Sebastian T, Yerramilli S, Lader E, Farrar WL, Hurt EM. The role of upregulated miRNAs and the identification of novel mRNA targets in prostatospheres. Genomics. 2012 Feb;99(2):108-17.
Urzúa U, Owens GA, Zhang GM, Cherry JM, Sharp JJ, Munroe DJ. Tumor and reproductive traits are linked by RNA metabolism genes in the mouse ovary: a transcriptome-phenotype association analysis. BMC Genomics. 2010 Dec 22;11 Suppl 5(Suppl 5):S1.
Roth MJ, Wei WQ, Baer J, Abnet CC, Wang GQ, Sternberg LR, Warner AC, Johnson LL, Lu N, Giffen CA, Dawsey SM, Qiao YL, Cherry J. Aryl hydrocarbon receptor expression is associated with a family history of upper gastrointestinal tract cancer in a high-risk population exposed to aromatic hydrocarbons. Cancer Epidemiol Biomarkers Prev. 2009 Sep;18(9):2391-6.
Althoff KN, Schlueter DJ, Anton-Culver H, Cherry J, Denny JC, Thomsen I, Karlson EW, Havers FP, Cicek MS, Thibodeau SN, Pinto LA, Lowy D, Malin BA, Ohno-Machado L, Williams C, Goldstein D, Kouame A, Ramirez A, Roman A, Sharpless NE, Gebo KA, Schully SD. Antibodies to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in All of Us Research Program Participants, 2 January to 18 March 2020. Clin Infect Dis. 2022 Mar 1;74(4):584-590.
- Monitors new developments in the biotechnology industry
- Translates new technology applications into useful applications for biomedical research
- Develop project-specific applications using state-of-the-art technologies
- Technology Transfer and consultation
- Offer formal and informal training, support, and troubleshooting
- Evaluate new technologies applications
- Established a new bioinformatics application that fully support investigators computational scientific needs
David H. McDermott, M.D.
The focus of our program is currently on the identification, diagnosis, and treatment of various primary immunodeficiencies especially as related to the chemotactic cytokine (chemokine) system which directs the migration, adhesion, activation, and function of leukocytes. We focus on WHIMS (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis Syndrome) which is due to autosomal dominant gain-of-function mutations in the CXCL12 chemokine receptor and HIV coreceptor known as CXCR4. We are repurposing a small molecule CXCR4 antagonist to treat this disorder and working on gene therapy to cure the disease as we have found that hematopoietic stem cells that lack one copy of CXCR4 have a selective bone marrow engraftment advantage. We are also working on a metabolic cause of severe congenital neutropenia and neutrophil dysfunction known as G6PC3 deficiency and attempting to repurpose empagliflozin to reduce the impact of a toxic metabolite of a dietary sugar that accumulates in leukocytes in this autosomal recessive immunodeficiency. Finally, in collaboration with Gigi Notarangelo’s group in NIAID, we recently discovered a novel immunodeficiency syndrome known as SASH3 immunodeficiency and are working on understanding the mechanism of the susceptibility to infectious diseases and autoimmunity that results from this X-linked recessive disorder.
A Phase 1 Study of Empagliflozin as a Treatment for Severe Congenital Neutropenia due to G6PC3 Deficiency: NCT05078879 Keywords: G6PC3
NIH Protocol #000236-I
A Phase III, Double-blind, Randomized, Crossover Study of Plerixafor Versus G-CSF in the Treatment of Patients with WHIM Syndrome: NCT02231879
Keywords: WHIM or WHIM syndrome
NIAID Protocol #14-I-0185
A Phase I Study of Mozobil in the Treatment of Patients with WHIMS: NCT00967785
Keywords: WHIM or WHIMS
NIAID Protocol #09-I-0200
Screening and Baseline Assessment of Patients with Abnormalities of Immune Function: NCT00128973
Keywords: immunodeficiency screening
NIAID Protocol #05-I-0213
Establishing Fibroblast-derived Cell Lines from Skin Biopsies of Patients with Immunodeficiency or Immunodysregulation Disorders: NCT00895271
Keywords:fibroblast biopsy
NIAID Protocol #09-I-0133
Delmonte OM, Bergerson JRE, Kawai T, Kuehn HS, McDermott DH, Cortese I, Zimmermann MT, Dobbs AK, Bosticardo M, Fink D, Majumdar S, Palterer B, Pala F, Dsouza NR, Pouzolles M, Taylor N, Calvo KR, Daley SR, Velez D, Agharahimi A, Myint-Hpu K, Dropulic LK, Lyons JJ, Holland SM, Freeman AF, Ghosh R, Similuk MB, Niemela JE, Stoddard J, Kuhns DB, Urrutia R, Rosenzweig SD, Walkiewicz MA, Murphy PM, Notarangelo LD. SASH3 variants cause a novel form of X-linked combined immunodeficiency with immune dysregulation. Blood. 2021 Sep 23;138(12):1019-1033.
Gao JL, Owusu-Ansah A, Paun A, Beacht K, Yim E, Siwicki M, Yang A, Liu Q, McDermott DH, Murphy PM. Low-level Cxcr4-haploinsufficient HSC engraftment is sufficient to correct leukopenia in WHIM syndrome mice. JCI Insight. 2019 Dec 19;4(24):e132140.
McDermott DH, Pastrana DV, Calvo KR, Pittaluga S, Velez D, Cho E, Liu Q, Trout HH 3rd, Neves JF, Gardner PJ, Bianchi DA, Blair EA, Landon EM, Silva SL, Buck CB, Murphy PM. Plerixafor for the Treatment of WHIM Syndrome. N Engl J Med. 2019 Jan 10;380(2):163-170.
McDermott DH, Gao JL, Liu Q, Siwicki M, Martens C, Jacobs P, Velez D, Yim E, Bryke CR, Hsu N, Dai Z, Marquesen MM, Stregevsky E, Kwatemaa N, Theobald N, Long Priel DA, Pittaluga S, Raffeld MA, Calvo KR, Maric I, Desmond R, Holmes KL, Kuhns DB, Balabanian K, Bachelerie F, Porcella SF, Malech HL, Murphy PM. Chromothriptic cure of WHIM syndrome. Cell. 2015 Feb 12;160(4):686-699.
McDermott DH, De Ravin SS, Jun HS, Liu Q, Priel DA, Noel P, Takemoto CM, Ojode T, Paul SM, Dunsmore KP, Hilligoss D, Marquesen M, Ulrick J, Kuhns DB, Chou JY, Malech HL, Murphy PM. Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood. 2010 Oct 14;116(15):2793-802.
McDermott DH, Zimmerman PA, Guignard F, Kleeberger CA, Leitman SF, Murphy PM. CCR5 promoter polymorphism and HIV-1 disease progression. Multicenter AIDS Cohort Study (MACS). Lancet. 1998 Sep 12;352(9131):866-70.
- Immunodeficiency / Neutropenia
- Immunogenetics of the Chemokine System
- Infectious Disease Susceptibility
- WHIMS (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis Syndrome)
- G6PC3 deficiency
- SASH3 deficiency

