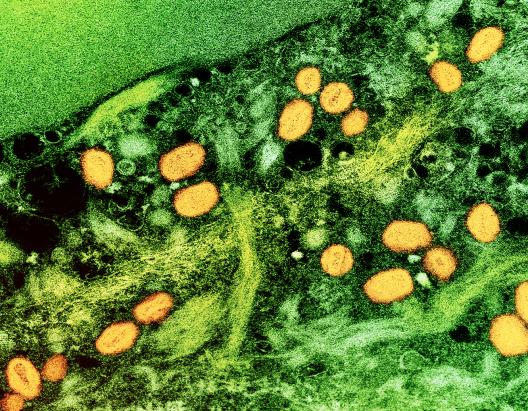
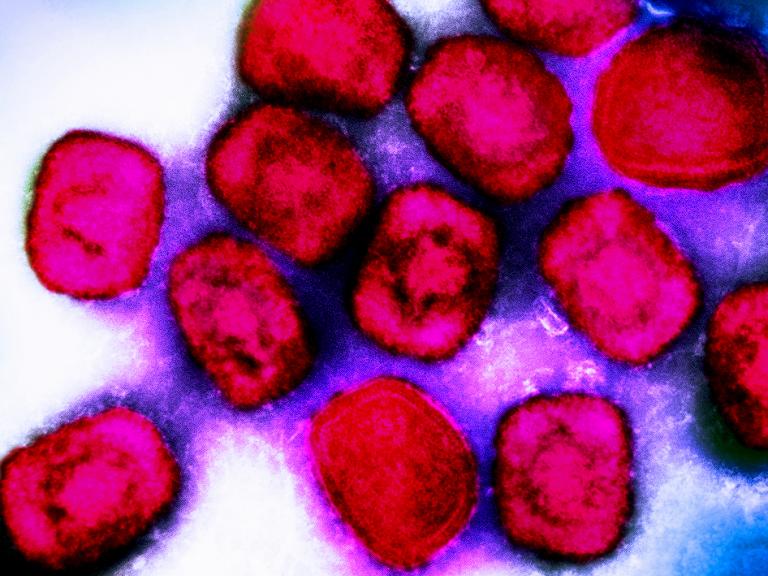
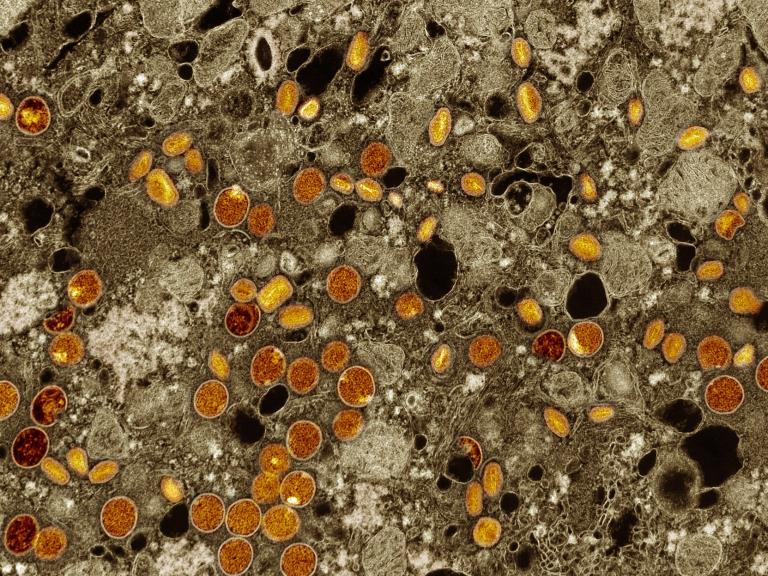
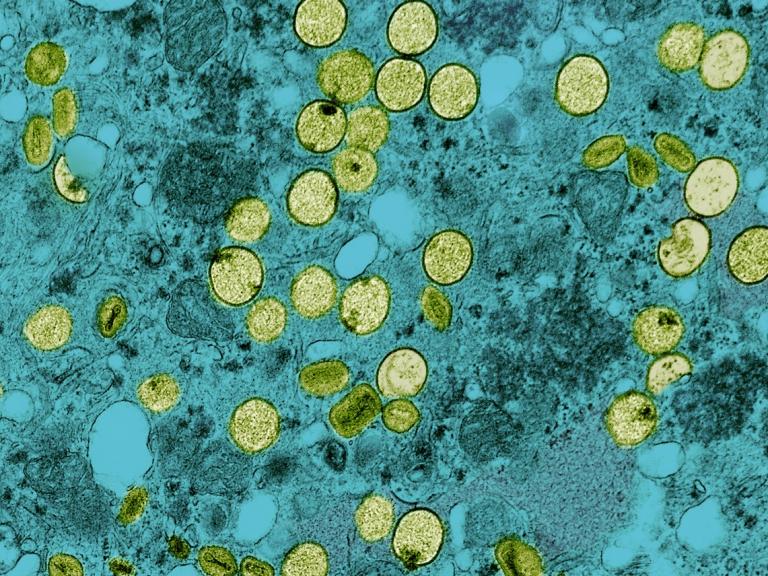
As part of the U.S. government response to the current mpox outbreak, the National Institutes of Health’s (NIH) National Institute of Allergy and Infectious Diseases (NIAID) released an update on its priorities for mpox research in September 2024.
Research
Research Areas
Grants & Contracts
Clinical Trials
News & Events
About NIAID
Website Policies and Notices