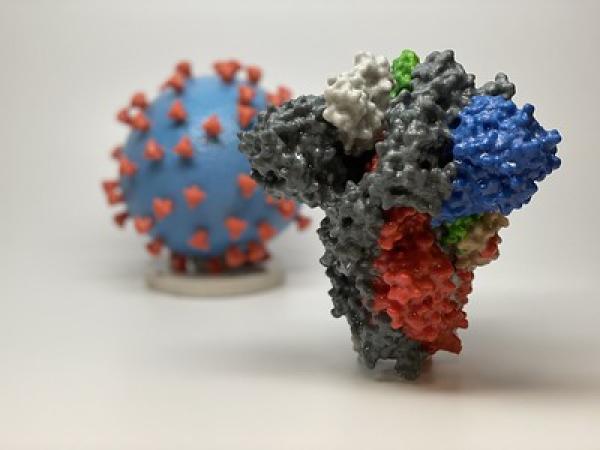
A 3-D printed model of a spike protein of SARS-CoV-2, the virus that causes COVID-19, in front of a 3D printed model of a SARS-CoV-2 virus particle. The spike protein (foreground) enables the virus to enter and infect human cells. On the virus model, the virus surface (blue) is covered with spike proteins (red) that enable the virus to enter and infect human cells.
Within hours of the public release of the viral genome sequence, scientists at the Vaccine Research Center (VRC) of the National Institute of Allergy and Infectious Diseases (NIAID) and their collaborators engineered a key protein from SARS-CoV-2, the virus that causes COVID-19, to enable its study as a vaccine candidate and for research applications. In the months that followed, NIAID, through its Technology Transfer and Intellectual Property Office (TTIPO), facilitated rapid distribution of the key protein to the global research community, enabling critical research and the global scientific response to the COVID-19 pandemic. Like other coronaviruses, SARS-CoV-2 particles are spherical and have proteins called spikes protruding from their surface. These spikes latch onto human cells, then allow the virus membrane to fuse with the human cell membrane. The viral genes can then enter the host cell to be copied, producing more viruses. Based on earlier work with SARS-CoV-1 and other coronaviruses, researchers at the VRC and collaborators were able to quickly engineer a version of SARS-CoV-2 with spike proteins stabilized in their prefusion conformation, which makes them more easily produced and a useful target for vaccine development relative to the native spike protein. Despite similarities between the spike proteins of SARS-CoV-1 and SARS-CoV-2, three different antibodies for the SARS-CoV-1 spike protein did not bind to the SARS-CoV-2 spike protein in tests. This early finding suggested that potential vaccine and antibody-based treatments would need to be specific to SARS-CoV-2. It also demonstrated the importance of rapid sharing of SARS-CoV-2 prefusion stabilized spike proteins, and the plasmid molecules that encode them, with researchers working to develop treatments and vaccines. As of October 8, 2020, NIAID had negotiated 83 Material Transfer Agreements (MTAs) with 70 academic organizations, non-profit entities, government agencies and other entities to provide SARS-CoV-2 prefusion stabilized spike proteins or plasmids for their research projects. Approximately 60% of the MTAs were signed within three days and more than 70% were completed within one week; nearly 80% were completed within two weeks. To further expedite sharing for research use worldwide, NIAID leveraged its Biodefense and Emerging Infections Research Resources Repository (“BEI Resources”) to produce and distribute the spike materials. Since June 2020 BEI Resources has fulfilled 55 requests for SARS-CoV-2 spike plasmids. In September 2020 TTIPO also signed an agreement with National Institute for Biological Standards and Control (NIBSC), a large repository in the U.K., to produce and distribute the materials. Additionally, 21 license agreements were executed with biotechnology and pharmaceutical companies for technologies related to SARS-CoV-2 prefusion stabilized spike proteins. Many of the licenses were signed within two weeks and the vast majority within one month. Most licensees planned to use the technology to support vaccine development.
Team Members:
Dr. Barney Graham
Dr. Kizzmekia Corbett
Dr. Amy Petrik
Dr. Carol Salata
Dr. Vincent Feliccia
Dr. Michael Mowatt
Judy Stein, MPH, MBA
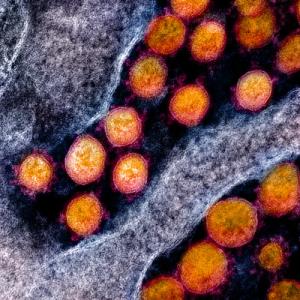
Transmission electron micrograph of SARS-CoV-2 virus particles, isolated from a patient. Image captured and color-enhanced by NIAID scientists at the Integrated Research Facility (IRF) in Fort Detrick, Maryland.
A clinical trial of remdesivir for COVID-19 patients, sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), wasn’t just the first trial of its kind in the U.S. In fact, findings from that trial led directly to two emergency use authorizations (EUAs) from the Food and Drug Administration (FDA) and ultimately to the FDA’s first approval of a COVID-19 therapy without an emergency use qualifier. Within weeks of the COVID-19 outbreak in Wuhan China, the Division of Microbiology and Infectious Diseases (DMID) at the NIAID began collaborating with companies to clinically assess existing therapies for treating COVID-19, utilizing networks supported by the National Institutes of Health as well as other sites. Remdesivir, an investigational antiviral drug developed by Gilead Sciences Inc., emerged as a promising early therapeutic candidate for COVID-19 based on preliminary studies involving other types of coronaviruses. On February 21, 2020—before the World Health Organization had declared a pandemic—DMID and NIAID initiated the Adaptive COVID-19 Treatment Trial (ACTT), a randomized, controlled clinical trial to evaluate the safety and efficacy of intravenous remdesivir in hospitalized adults diagnosed with COVID- 19. This NIAID-sponsored trial was the first clinical trial in the U.S. to evaluate an experimental treatment for COVID-19. The Technology Transfer and Intellectual Property Office (TTIPO) at NIAID worked with DMID to negotiate a Clinical Trial Agreement (CTA) with Gilead to obtain remdesivir and draft template agreements to expedite expanded testing within the United States and internationally. The creative technology transfer solutions and expeditiously negotiated agreements enabled initiation of the ACTT trial less than two months after identification of the virus responsible for the initial Wuhan outbreak. The FDA issued an EUA on May 1, 2020 for the emergency use of remdesivir to treat hospitalized patients with severe COVID-19, and on August 28 expanded the EUA by no longer limiting its use to patients with severe disease. On October 22, the FDA approved remdesivir for the treatment of most COVID-19 patients that require hospitalization, the first therapy to receive a non-EUA approval for COVID-19 use. The ACTT trial has progressed to test remdesivir in combination with other existing therapies. ACTT-2 began on May 8 to test remdesivir plus Eli Lilly’s anti-inflammatory drug baricitinib. ACTT-3 began on August 5 to assess remdesivir plus Merck’s immunomodulatory drug interferon-beta 1a. For those two trials, the NIAID TTIPO successfully negotiated CTAs with Eli Lilly to obtain baricitinib and with EMD Serono (d.b.a. Merck) to obtain interferon beta-1a. NIAID is pursuing testing of potential compounds in combination with remdesivir under NIH’s Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) program, and the successful combinations will be further evaluated in larger clinical trials, potentially leading to additional robust and effective therapies for COVID-19.
Team Members:
Dr. Yogikala Prabhu
Mr. Ahsen Khan
Dr. Richard Williams

A vial from Washington State SARS-CoV-2 isolate was shared through the Biodefense and Emerging Infections (BEI) Resources, a repository supported by NIAID. The first SARS-CoV-2 case in the U.S. was announced by the state of Washington on January 21, 2020. The isolate was received from CDC and appeared in the BEI catalog on February 6, 2020.
Collaboration among three Health and Human Services (HHS) agencies enabled rapid sharing of viral materials to accelerate the scientific and medical responses to the coronavirus disease 2019 (COVID-19) pandemic. In early 2020, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as a novel virus and tracked around the world. It became apparent that scientists knew little about the basic biology of SARS-CoV-2 or how to treat the resulting infectious disease now known as COVID-19. Rapid sharing of SARS-CoV-2 materials, especially SARS-CoV-2 virus strains, thus became essential to improve the general understanding of the virus and support the development of effective diagnostics, treatments, and vaccines. Academic centers, U.S. government agencies, private companies, and the public health community requested specimens from National Institute of Allergy and Infectious Diseases (NIAID) to support SARS-CoV-2 research and development of medical countermeasures. Fortunately, the HHS agencies previously developed a strategy for sharing viral and biological samples during the Zika virus epidemic in 2016. Since that time, the NIAID, Centers for Disease Control and Prevention (CDC), and HHS Office of Global Affairs developed a more efficient and collaborative approach for sharing critical viral materials. Central to this effort, two mechanisms developed during the 2016 Zika outbreak were adapted to the COVID-19 pandemic. First, interagency partners developed a streamlined Materials Transfer Agreement for use in emergency situations. This Emergency Use Simple Letter Agreement (EUSLA) allows the materials to be used for any legitimate purpose required to rapidly prevent, detect, prepare for, and respond to the spread or transmission of SARS-CoV-2, including commercial development. Second, the team used a NIAID-supported biorepository to receive, grow, validate, and distribute viral materials. CDC reached out to its extensive global network of partner laboratories and sentinel surveillance sites to access SARS-CoV-2 samples in early February 2020. Similarly, NIAID was able to access samples from around the world by communicating through its grantee and contractor network. The volume of transfers documented, and the diverse nature of the recipients attest to the success of this strategy:
- 22 organizations contributed materials to the biorepository
- 8,441 requests for materials fulfilled, all under EUSLA
- 4,108 agreements negotiated with 319 academic institutions, 509 companies, 39 federal and state agencies and 5 foreign governments
- Materials distributed to 49 states in the US and Puerto Rico and 43 countries
Team Members:
CDC:
Dr. Natalie Thornburg
Dr. Wendi Kuhnert-Tallman
Kevin Brand
Marie-Christine Reames
NIAID:
Dr. Mukul Ranjan
Dr. Michael Mowatt
Dr. Peter Tung
Dr. Alan Embry
Ms. Kimberly Stemple
Dr. Marciela Maria De Grace
Dr. Brooke Bozik
HHS:
Dr. Robin Moudy
Ruvani Chandrasekera
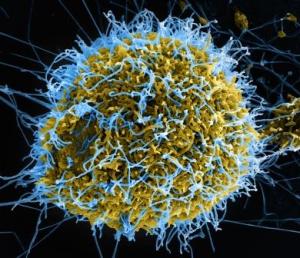
Image of a Colorized scanning electron micrograph of filamentous Ebola virus particles budding from a chronically infected VERO E6 cell.
In 2014, as the Ebola outbreak escalated, NIAID collaborated with pharmaceutical company GlaxoSmithKline (GSK) on NIAID-planned clinical trials to test Ebola vaccine candidates. Their collaboration exemplifies the flexibility and creativity of the NIAID Technology Transfer and Intellectual Property Office (TTIPO), who developed unique and expedited agreements to facilitate the development of an Ebola vaccine.
Researchers at the NIAID Vaccine Research Center (VRC) had already been developing and testing ebolavirus vaccine candidates for over 15 years. In 2008, a research collaboration agreement (RCA) allowed VRC to work with Okairos, a Switzerland-based biotechnology company specializing in vaccine platform technologies. Vaccine platforms are the ways in which a vaccine gets delivered to the immune system. The RCA was to use one of Okairos’ platforms, a chimpanzee adenovirus vector (cAd), to deliver the VRC’s candidate ebolavirus (EBOV) glycoprotein gene, creating the cAd3-EBOV vaccine. The approach resulted in an effective immune response to EBOV glycoproteins in humans.
In 2013, GSK acquired Okairos and its RCA with NIAID, and in 2014, GSK and NIAID expanded upon the agreement to better respond to the Ebola outbreak. TTIPO worked on the time-sensitive agreements for this collaboration: All agreements needed for the start of cAd3-EBOV vaccine clinical trials were negotiated and executed between September 2014 and early 2015. Unique agreements were drafted to circumvent anticipated delays that could have slowed the start of clinical trials, including a restrictive material transfer agreement (MTA) that was used to send cAd3-EBOV test particles to the clinical trials sites in the United Kingdom, enabling it to clear customs and make it to the in time for labeling.
This nimble work allowed the clinical trial to begin within hours of the execution of the clinical trial agreement, ensuring that no valuable time was lost waiting to clear red tape. GSK has continued the development of the cAd3-EBOV vaccine candidates in Phase II trials in Africa.
The Federal Laboratory Consortium for Technology Transfer (FLC) recognized NIAID with an Excellence in Technology Transfer Award for their work on unique and expedited agreements needed for Ebola vaccine development after the 2014 Ebola outbreak. Congratulations to Nancy Sullivan, Vincent Feliccia, Carol Salata, Amy Petrik, Judy Stein, and Rosemary Walsh for this recognition.
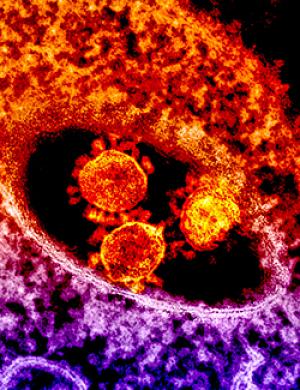
The round, spiked objects at center are MERS coronavirus particles.
The 2016 Federal Laboratories Consortium (FLC) planner features two new NIAID technologies.
One is a mobile phone video microscope from the NIAID Laboratory of Parasitic Diseases and the University of California, Berkeley. The video microscope can measure blood levels of the parasitic filarial worm Loa loa, which causes river blindness and elephantiasis. This technology enables quick quantification of parasites in the blood sample of just a fingerprick, assisting public health efforts to eradicate the neglected tropical diseases that have such a profound impact on quality of life in resource-poor countries.
The other featured technology comes from the Virus Ecology Unit, NIAID Laboratory of Virology, whose study of the novel Middle East respiratory syndrome-coronavirus (MERS-CoV) has uncovered a promising new animal model for research. Using computer simulations, scientists predicted that marmosets could be infected with MERS-CoV. Then in studies, they discovered that MERS-CoV infection in marmosets closely mimics infection in humans, providing the best animal model yet to test potential therapies.
There were 135 submissions for the 2016 FLC Planner, which will go out to more than 12,000 FLC members, as well as members of industry and academia. The planner supports the FLC mission to promote and facilitate the rapid movement of federal laboratory research results and technologies into the mainstream of the U.S. economy.
The 2015 Planner for the Federal Laboratories Consortium (FLC) will feature two NIAID technologies. One is an Ebola vaccine antibody study from Heinz Feldmann, M.D., Ph.D., chief of the Laboratory of Virology at the NIAID Rocky Mountain Laboratories. NIAID researchers and colleagues determined that the level of antibody production was the critical factor for protection in experimental vaccines against Ebola virus.
The other featured technology comes from NIAID Director Anthony S. Fauci, M.D., discovering the source of the memory B cells that produce rare, neutralizing HIV antibodies in a subset of early infected patients. The findings augment the current understanding of how HIV disease develops and has implications for the timing of treatment.
There were 118 submissions for the 2015 FLC Planner, which will go out to more than 12,000 FLC members, as well as members of industry and academia. The planner supports the FLC mission to promote and facilitate the rapid movement of federal laboratory research results and technologies into the mainstream of the U.S. economy.
See the June entry of the 2015 FLC Planner featuring an NIAID Ebola vaccine antibody study.
See the planner's Featured Technologies showcasing NIAID's Timing of HIV Treatments.
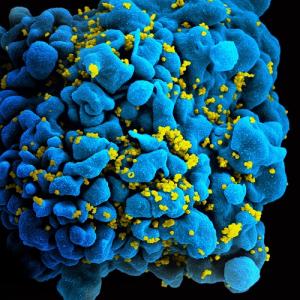
Scanning electromicrograph of an HIV-infected T cell.
The Federal Laboratory Consortium for Technology Transfer (FLC) recently recognized NIAID with a Mid-Atlantic Regional Award for Excellence in Technology Transfer for novel therapeutics to treat malaria. The therapeutics were developed out of an NIAID discovery about how the malaria parasite nourishes itself.
Led by Sanjay Desai, M.D., Ph.D., the Apicomplexan Molecular Physiology Section in the Laboratory of Malaria and Vector Research, NIAID Division of Intramural Research, discovered the the malaria parasite’s plasmodial surface anion channel (PSAC). The parasite uses PSAC to take in nutrients from infected blood cells and so is an important target for anti-malaria drugs.
The therapeutics were developed through several collaborations, including a conditional gift from the Medicines for Malaria Venture, as well as a cooperative research and development agreement (CRADA) and small business innovation research (SBIR) grant with biopharmaceutical company Microbiotix.
Congratulations to Sanjay, his team, and the Technology Transfer and Intellectual Property Office for this recognition.
For more information about PSAC, watch this video.

From left to right: Michael R. Mowatt, Ph.D.; Kevin Chang, Ph.D.; Sanjay Desai, M.D., Ph.D.; Mukul Ranjan, Ph.D.; Dana Hsu, J.D, M.S.; Not pictured: Charles Rainwater, J.D., M.B.A.

The sound attenuation canopy installed.
The Federal Laboratory Consortium for Technology Transfer (FLC) recently recognized NIAID with two national Awards for Excellence in Technology Transfer, for a novel therapeutic for tuberculosis, called SQ109, and for a sound attenuation canopy that reduces office noise. SQ109 arose from research in the Tuberculosis Research Section, headed by Clifton E. Barry III, Ph.D., of the Division of Intramural Research, Laboratory of Clinical Infectious Diseases. SQ109 is currently licensed to Sequella, Inc., and is in Phase II clinical trials for the treatment of pulmonary tuberculosis.
The sound attenuation canopy was developed by staff in the Office of Research Operations, NIAID Office of Science Management and Operations, headed by Judy Quasney. This product diminishes sound travel through office ceilings while still allowing air flow. Over 500 devices, in experimental form, were installed in Building 6610 on all floors. Data collected from these devices was used to help refine the product, which will be used in NIAID’s new Fishers Lane building. The sound attenuation canopy previously won the Mid-Atlantic FLC Excellence in Technology Transfer Award.
Read more about the sound attenuation canopy in Muting Workplace Noise.

Left to right: Clifton E. Barry III, Ph.D.; Mukul Ranjan, Ph.D.; Tristan Mahyera, M.S., J.D.; Meghan Van Horn, M.S.,J.D; and Michael R. Mowatt, Ph.D. Not pictured: Edward Fenn
Diagnostic Test Is Based on Technology From NIAID Lab
In 2004, diagnostics manufacturer R-Biopharm licensed recombinant baculoviruses developed in the NIAID Laboratory of Infectious Diseases and used the virus-derived virus-like particles (VLPs) to create monoclonal antibodies against norovirus. In R-Biopharm’s Ridascreen test, these antibodies serve to capture norovirus antigen from a stool sample. If the antigen is present, the antibodies bind to it so it can be detected in later steps of the test. The Food and Drug Administration (FDA) approved the Ridascreen Norovirus test for diagnosing norovirus infection in situations where a number of people have simultaneously contracted gastroenteritis and there is a clear potential avenue for virus transmission such as a shared location or food. This is the first norovirus diagnostic approved by FDA.
Read more about Ridascreen and NIAID’s role in NIAID-Developed Technology Key in First FDA-Approved Norovirus Test. The story was also featured in the 2012 Technology for Today, a publication from the Federal Laboratory Consortium, on page 24.

Shown are (from l) J. Scott Deiter, Chair, FLC, and Director of Technology Transfer, Naval Surface Warfare Center, Indian Head, Maryland; Arnold Kirshenbaum, M.D., Adjunct Investigator, Laboratory of Allergic Diseases, NIAID; Michael R. Mowatt, Ph.D., Dir
On May 7, 2009, NIAID received an Award for Excellence in Technology Transfer from the Federal Laboratory Consortium for Technology Transfer (FLC) for the highly effective transfer of a “Mast Cell Line for Research on Allergies and Inflammatory Diseases.” Developed by Arnold Kirshenbaum, M.D.; Cem Akin, M.D., Ph.D.; and Dean Metcalfe, M.D., the line represents a potent tool for understanding the normal functions of human mast cells and identifying the mechanisms of a variety of diseases. Research using this cell line holds great promise in the development of novel therapies to combat allergic diseases.
Read more on the front page of the June 2009 issue of FLC NewsLink.
NIAID, Merial Use Poxvirus Technology To Halt the Spread of Disease in the Wild
Merial has developed an innovative oral rabies vaccine targeting wildlife. The vaccine uses the poxvirus as a vector—a technology developed in NIAID labs. Approximately 12 million doses of Raboral V-RG are distributed in the wild each year. Read more on page 6 of the December 2008 issue of FLC NewsLink
The hepatitis A virus, an enterically transmitted pathogen that has infected an estimated 5 billion of the world’s population, is the single most important cause of acute clinical hepatitis in the United States. GlaxoSmithKline (formerly SmithKline Beecham) initiated a CRADA in 1986 with NIAID's Laboratory of Infectious Diseases, Hepatitis Viruses Section, to develop an inactivated vaccine for the hepatitis A virus. As a result, GlaxoSmithKline licensed the vaccine technology in Europe in 1991 and in the United States in 1995, marketing a vaccine under the brand name Havrix. In addition, the parties have collaborated under CRADAs to develop vaccines against hepatitis C and E viruses.
MedImmune, Inc., markets this humanized monoclonal antibody for the prevention of serious lower respiratory tract disease caused by RSV in high-risk infants and children. This successful product is based on a murine monoclonal antibody invented by NIAID and MedImmune scientists. MedImmune acquired commercialization rights through a license with the National Institutes of Health.
NIAID Key Player in Partnership To Prevent Pneumococcal Disease
Pneumonia is the major cause of death in Gambian children, with Streptococcus pneumoniae as the leading bacterial cause. In 2000, NIAID negotiated a CRADA with Wyeth to supply an investigational pneumococcal conjugate vaccine for a clinical trial that was jointly funded by NIAID and several international health organizations. The trial recruited approximately 17,000 Gambian children shortly after birth and monitored them for 30 months to measure the ability of the vaccine to prevent X-ray-confirmed pneumonia and other forms of serious pneumococcal disease. Results show that the vaccine substantially reduces the incidence of invasive pneumococcal disease and pneumonia in young children. The vaccine group had 16 percent fewer deaths from any cause, indicating the potential value of the vaccine in overall child survival in developing countries.
NIAID and GSK Team Up To Thwart Endemic Virus
The Pakistani strain of hepatitis E virus has the potential to be developed into a hepatitis vaccine for people in areas where hepatitis E is endemic (Southeast and Central Asia and parts of Africa). An experimental vaccine, resulting from a CRADA between GlaxoSmithKline and NIAID, was highly efficacious in preclinical evaluations, and a recently completed field trial of the vaccine in Nepal confirmed that it protects against hepatitis E in at-risk populations.
NIAID and Chiron Test Strategy To Prevent or Delay AIDS Onset
Beginning in 1999, two large, multi-site, international Phase III studies were independently designed and developed to address the question of whether IL-2 therapy confers long-term clinical benefit to people with HIV infection. The first study evaluates the safety and clinical efficacy of intermittent cycles of recombinant interleukin-2 (rIL-2; Proleukin) in combination with standard antiretroviral therapy, compared with standard antiretroviral therapy alone, in almost 2,000 patients with advanced HIV infection. The second study, conducted in part under a CRADA with the Chiron Corporation, evaluates a similar rIL-2 treatment strategy in over 4,000 patients with earlier HIV disease. Over the next several years, each study is expected to provide an independent answer to the question of whether intermittent IL-2 therapy can help prevent or delay the onset of AIDS to a greater degree than is currently possible with the combinations of conventional antiretroviral medications available.