Mpox is caused by the monkeypox virus (MPXV). MPXV is part of the Orthopoxvirus genus, which also includes variola virus (the cause of smallpox), vaccinia virus, and cowpox virus. It causes mpox disease, formerly known as monkeypox disease. Although mpox is similar to smallpox, it is much less deadly. Initial symptoms of mpox include: fever, headache and body aches, fatigue, and swollen lymph nodes, followed by a rash affecting the skin and often mouth or genital area. Human-to-human transmission of the virus occurs through direct contact with lesions or body fluids, prolonged close contact including sexual contact, and indirect contact with contaminated clothing or bedding.
The first human case of mpox was recorded in 1970 in the Democratic Republic of the Congo (DRC). The disease is endemic in central and western Africa. Two types of the virus that causes mpox have been identified. Clade I is endemic in Central Africa and can cause severe illness. Clade II, historically endemic in West Africa, tends to result in milder illness. People with compromised immune systems, children, and people who are pregnant are especially vulnerable to severe mpox regardless of the virus clade.
NIAID is conducting and supporting research focused on developing and evaluating treatments and vaccines for mpox, understanding disease pathogenesis, transmission, and spillover, evaluating immunological responses to MPXV, and bolstering the critical research resources foundational to supporting the ongoing public health response.
Editorial Note: The term “monkeypox” was previously used to describe the clinical disease caused by MPXV. The term “mpox” is preferred and now widely adopted because it is less stigmatizing. The virus that causes mpox is still classified as monkeypox virus (MPXV) by the International Committee on Taxonomy of Viruses.
Related Public Health and Government Information
For information on the status outbreaks and mpox in the United States, please visit the Centers for Disease Control and Prevention mpox page.
For information on the regulatory status of mpox vaccines and treatment in the United States, please consult the Food and Drug Administration.
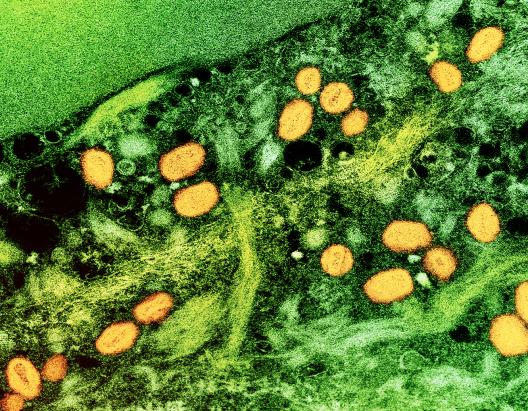
Highlights
Sequencing of Congo Mpox Reports Highlights New Transmission Patterns in Country
Laboratory analysis in the Republic of Congo showed mpox was affecting people in parts of the country where it has not been historically. The findings point to increases in human-to-human transmission across the border with the neighboring Democratic Republic of the Congo, where a large outbreak was declared a public health emergency of international concern.
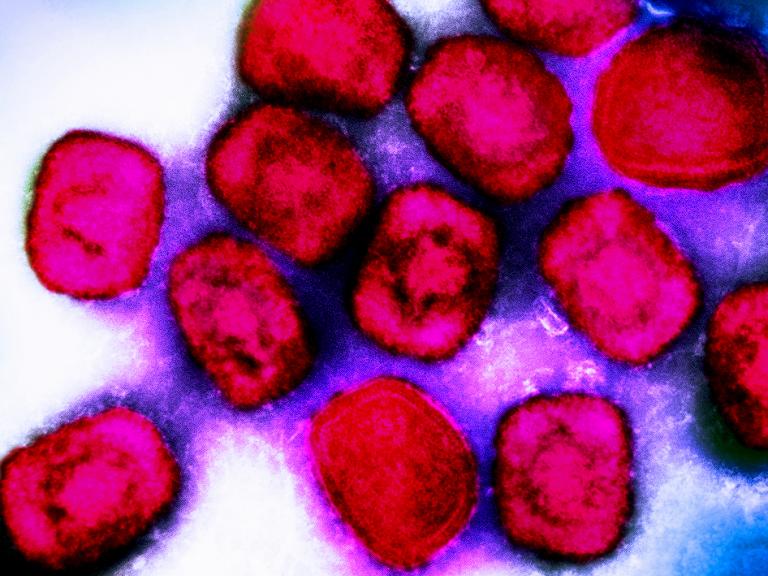
Antiviral Tecovirimat is Safe but Did Not Improve Clade I Mpox Resolution in DRC
Initial analysis of data from PALM007 trial showed the antiviral drug tecovirimat did not reduce the duration of mpox lesions among children and adults with clade I mpox in the Democratic Republic of the Congo.
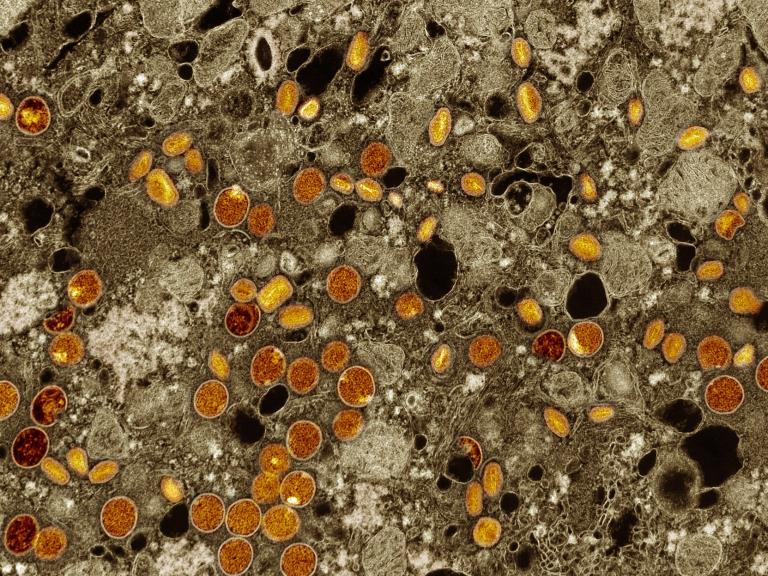
Mpox Vaccine Is Safe and Generates a Robust Antibody Response in Adolescents
A clinical trial of an mpox vaccine in adolescents found it was safe and generated an antibody response equivalent to that seen in adults. Results were presented at IDWeek2024.

