Malaria is one of the most widespread and devastating infectious diseases across the globe. This mosquito-borne parasitic disease killed approximately 619,000 people in 2021 alone, many of them children in Africa. In one of the deadliest forms of malaria, known as cerebral malaria, the patient experiences severe neurological symptoms, such as seizures and coma. Although only a small fraction of people who fall ill with malaria also experience cerebral malaria, the condition is lethal without treatment. Among hospitalized patients with the condition, death rates range between 15 and 20%. In a new paper, recently published in Science Translational Medicine, researchers from the National Institute of Allergy and Infectious Diseases (NIAID), part of the NIH, and their colleagues studied children with cerebral malaria in Malawi to better understand the underlying causes of these devastating symptoms in the hope of developing improved treatments.
Researchers know that the symptoms of cerebral malaria are caused when the brain swells within the confines of the skull, eventually impinging upon the brainstem, which causes breathing to stop. However, researchers have been unsure how malaria infection leads to brain swelling. Some researchers hypothesized that the main cause was a weakening of the blood-brain barrier, which would allow fluid to seep into the brain and cause it to swell. Others speculated that the primary driver behind the swelling was inside the blood vessels themselves. Red blood cells infected with P. falciparum, the parasite which causes malaria, can become “sticky,” adhering to the walls of blood vessels. Partial blockages inside the cerebral veins could slow the flow of blood leaving the brain, causing the blood vessels themselves to become engorged and expand the brain from within.
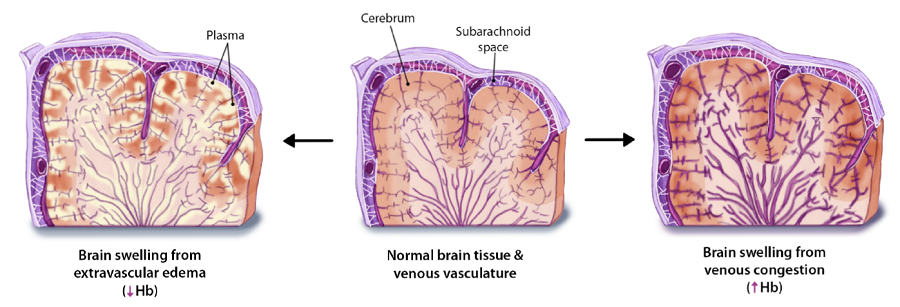
This illustration shows two different ways that cerebral malaria could cause brain swelling: fluid seeping into the brain (extravascular edema) or swollen blood vessels (venous congestion.)
To distinguish between these two hypotheses, NIAID researchers and their collaborators used non-invasive imaging techniques to study the flow of blood within the brains of 46 children who had been hospitalized for cerebral malaria at the Pediatric Research Ward of Queen Elizabeth Central Hospital in Blantyre, Malawi. As a comparison, they also studied 33 children with uncomplicated malaria and 26 healthy children from the local region. By using a light-based external monitoring tool (called near-infrared spectroscopy, or NIRS) the researchers were able to measure the amount of hemoglobin in the children’s brains. They reasoned that if excess fluid was the cause of brain swelling, then the hemoglobin concentration would be low, due to dilution. Alternatively, if the blood vessels were engorged with blood, then the hemoglobin concentration would be high.
The researchers found that children with cerebral malaria had significantly higher concentrations of hemoglobin inside their brains than children who had uncomplicated malaria, or healthy children—and among those with cerebral malaria, higher hemoglobin concentrations were correlated with greater brain swelling. In addition, hemoglobin concentrations fluctuated more unpredictably in children with cerebral malaria—suggesting that the normal mechanisms for controlling blood flow in the brain were disturbed. Together, these results support the hypothesis that obstruction to the outflow of blood from the brain, likely because the blood vessels themselves are clogged by red blood cells infected with P. falciparum adhering to the walls, is a main driver of brain swelling in patients with cerebral malaria.
The researchers say that these results may lead to a better understanding of why some treatments for cerebral malaria are not as effective as expected. For instance, steroids or osmotic agents are sometimes used to treat brain swelling by reducing the leakage of fluid from the blood vessels into the brain—but if the swelling is caused by the parasite-related effects within the blood vessels themselves, these treatments would not address the underlying problem. By pinpointing the exact mechanism by which cerebral malaria leads to brain damage, the researchers hope, we may improve treating it.
Reference: R L Smith et al. Increased brain microvascular hemoglobin concentrations in children with cerebral malaria. Science Translational Medicine DOI: 10.1126/scitranslmed.adh4293 (2023)