Vector Molecular Biology Section
Established in 2002
Jesus G. Valenzuela, Ph.D.
Chief, Vector Molecular Biology Section
Deputy Chief, Laboratory of Malaria and Vector Research

Highlights
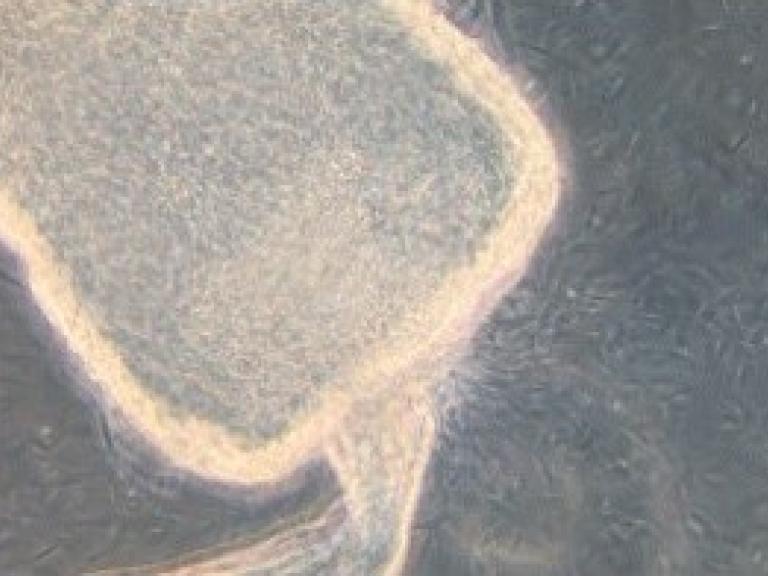
Battling Blood-Sucking Bugs
The NIH Intramural Research Program features Jesus Valenzuela's research dissecting how our bodies react to insect bites.
The image on the left shows the parasites that cause leishmaniasis coming out of a sand fly's gut, where they undergo a key part of their life cycle before being transmitted to a human via the fly's bite.
Major Areas of Research
- Investigating human skin immune responses to tick and insect bites
- Developing diagnostic tools to assess prior exposure to tick and insect bites in humans
- Identifying blood components that influence parasite infection and genetic exchange within the insect gut
- Studying the mechanism of how we sense ticks and insects
Program Description
The Vector Molecular Biology Section investigates immune responses to ticks and insect bites, focusing on acquired cellular immunity against vector arthropods. Our research examines how these immune responses help humans detect and remove ticks before they transmit pathogens. Additionally, we study how adaptive immune responses impact pathogen transmission by insects, with the goal of developing interventions to prevent insect-borne diseases and parasitic infections.
Our primary focus is on ticks, sand flies, and mosquitoes, with ticks posing a significant public health threat in the U.S. and globally. Ticks transmit various diseases to humans, pets, and livestock, including Lyme disease, Rocky Mountain spotted fever, anaplasmosis, and the Powassan virus. In the U.S., ticks surpass mosquitoes as the leading disease vector for humans and animals. Beyond infectious diseases, ticks can also trigger conditions such as red meat allergy and tick paralysis.
The section employs a multidisciplinary approach, combining immunology, biochemistry, RNA sequencing, bioinformatics, functional genomics, fieldwork, and clinical studies. One key area of research focuses on the initial immunological events in the skin after a tick or insect bite, using this knowledge to prevent pathogen transmission. Another area explores how arthropod vectors become infectious, leading to groundbreaking discoveries, such as a new developmental stage of Leishmania in the sand fly gut and the role of natural antibody IgM in parasite genetic exchange.
A critical area of ongoing research is the development of diagnostic tools to assess prior exposure to tick and insect bites in humans. These tools use salivary proteins or recombinant proteins from ticks and insects to detect antibodies in exposed individuals. Such diagnostic innovations guide vector control strategies and preventive measures. Together, these advancements aim to identify at-risk individuals and protect them from tick-borne illnesses.
Biography
Education
Ph.D., 1995, University of Arizona
Dr. Valenzuela received his Ph.D. in biochemistry from the University of Arizona in 1995. He joined the Laboratory of Parasitic Diseases in 1996, became a research fellow in 1999, and became a tenure-track investigator in the Laboratory of Malaria and Vector Research in October 2002. Dr. Valenzuela became a Senior Investigator in October 2009. In 2019, Dr. Valenzuela became deputy chief of the Laboratory of Malaria and Vector Research.
Clinical Studies
- Defining Skin Immunity of a Bite of Key Insect Vectors in Humans; ClinicalTrials.gov Identifier: NCT03641339
- Human Immune Response to Ixodes Scapularis Tick Bite: NCT05036707
- Evaluating the Safety and Immunogenicity of AGS-v PLUS, a Universal Mosquito-Borne Disease and Mosquito Control Vaccine: NCT04009824
- Safety and Immunogenicity of a First-in-Human Mosquito Saliva Peptide Vaccine; ClinicalTrials.gov Identifier: NCT03055000
Selected Publications
Chea S, Willen L, Nhek S, Ly P, Tang K, Oristian J, Salas-Carrillo R, Ponce A, Leon PCV, Kong D, Ly S, Sath R, Lon C, Leang R, Huy R, Yek C, Valenzuela JG, Calvo E, Manning JE, Oliveira F. Antibodies to Aedes aegypti D7L salivary proteins as a new serological tool to estimate human exposure to Aedes mosquitoes. Front Immunol. 2024 May 1;15:1368066.
de Araujo FF, Abdeladhim M, Teixeira C, Hummer K, Wilkerson MD, Ressner R, Lakhal-Naouar I, Ellis MW, Meneses C, Nurmukhambetova S, Gomes R, Tolbert WD, Turiansky GW, Pazgier M, Oliveira F, Valenzuela JG, Kamhawi S, Aronson N. Immune response profiles from humans experimentally exposed to Phlebotomus duboscqi bites. Front Immunol. 2024 Apr 3;15:1335307.
Serafim TD, Iniguez E, Barletta ABF, Cecilio P, Doehl JSP, Short M, Lack J, Nair V, Disotuar M, Wilson T, Coutinho-Abreu IV, Meneses C, Andersen J, Alves E Silva TL, Oliveira F, Vega-Rodriguez J, Barillas-Mury C, Ribeiro JMC, Beverley SM, Kamhawi S, Valenzuela JG. Leishmania genetic exchange is mediated by IgM natural antibodies. Nature. 2023 Nov;623(7985):149-156.
Guimaraes-Costa AB, Shannon JP, Waclawiak I, Oliveira J, Meneses C, de Castro W, Wen X, Brzostowski J, Serafim TD, Andersen JF, Hickman HD, Kamhawi S, Valenzuela JG, Oliveira F. A sand fly salivary protein acts as a neutrophil chemoattractant. Nat Commun. 2021 May 28;12(1):3213.
Serafim TD, Coutinho-Abreu IV, Oliveira F, Meneses C, Kamhawi S, Valenzuela JG. Sequential blood meals promote Leishmania replication and reverse metacyclogenesis augmenting vector infectivity. Nat Microbiol. 2018 May;3(5):548-555.
Anderson JM, Moore IN, Nagata BM, Ribeiro JMC, Valenzuela JG, Sonenshine DE. Ticks, Ixodes scapularis, Feed Repeatedly on White-Footed Mice despite Strong Inflammatory Response: An Expanding Paradigm for Understanding Tick-Host Interactions. Front Immunol. 2017 Dec 18;8:1784.
Articles
- Survival of the Fittest Parasite
- Leishmania Parasite Uses Host Antibodies in Insect Vector’s Blood Meal to Breed
Patents
- Valenzuela JG, Belkaid Y, Kamhawi S, Sacks D, Ribeiro JMC, inventors; The United States of America as represented by the Secretary of the Department of Health and Human Services, assignee. Anti-arthropod vector vaccines, methods of selecting and uses thereof. United States patent US 7,964,576. 21 Jun 2011.
- Valenzuela JG, Ribeiro JMC, Kamhawi S, Belkaid Y, Fischer L, Audonnet JC, Milward F, inventors; The United States of America as represented by the Department of Health and Human Services, Merial Limited, assignees. P. ariasi polypeptides, p. perniciosuspolypeptides and methods of use. United States patent US 7,741,437. 22 Jun 2010.
- Valenzuela JG, Ribeiro JMC, Barral A, Netto M, Brodskyn C, Gomes R, inventors; The United States of America as represented by the Secretary of the Department of Health and Human Services, Cruz, assignees. Lutzomyia longipalpis polypeptides and methods of use. United States patent US 7,485,306. 3 Feb 2009.
- Valenzuela JG, Belkaid Y, Kamhawi S, Sacks D, Ribeiro JMC, inventors; The United States of America as represented by the Department of Health and Human Services, assignee. Anti-arthropod vector vaccines, methods of selecting and uses thereof. United States patent US 7,388,089. 17 Jun 2008.
- Ribeiro JMC, Valenzuela JG, Charlab R, Mather TN, inventors; The United States of America as represented by the Department of Health and Human Services, University of Rhode Island, assignees. Ixodes salivary anticomplement protein. United States patent US 7,153,947. 26 Dec 2006.
- Francischetti IMB, Valenzuela JG, Ribeiro JMC, inventors; The United States of America as represented by the Department of Health and Human Services, assignee. Ixodes scapularis tissue factor pathway inhibitor. United States patent US 7,078,508. 18 Jul 2006.
Visit the U.S. Patent and Trademark Office for a complete patent listing.
Participating Research Networks
- Pfizer, Johns Hopkins University, University of Florida, Yale University
- GHIT partnership with CBER, FDA; Ohio State University, McGill University, Gennova Biopharmaceuticals Limited and Nagasaki University
- Yale School of Medicine
- Yale School of Public Health
- University of Maryland School of Medicine
- Uniformed Services University of the Health Sciences
- Federal University of Minas Gerais
- Federal University of Ouro Preto
- Liverpool School of Tropical Medicine
- Centro de Inv. en Alimentación y Desarrollo, A.C. (CIAD)
Research Group
The Vector Molecular Biology Section focuses on understanding how molecules from arthropod vectors are critical for the success of pathogen transmission and translating this knowledge into disease control opportunities.


