Vector Molecular Biology Section
Shaden Kamhawi, Ph.D.
Group Leader, Insect-Human-Pathogen and Insect-Reservoir-Pathogen Interactions, Vector Molecular Biology Section

Major Areas of Research
- Development of diagnostics tools to detect exposure to pathogens, insects, and ticks
- The host immune response to insect-borne pathogens
- Pathogen-insect interactions
- Field expertise in epidemiology and pathogen transmission of insect-borne diseases
Program Description
The research program is focused on basic and translational aspects of insect-borne diseases, with a focus on leishmaniasis. Leishmaniasis is an infectious disease endemic in southern states, including Texas and Oklahoma, and is also prevalent in foxhound dogs throughout the United States. Importantly, leishmaniasis has been one of the major afflictions in U.S. soldiers and military working dogs (MWD) deployed to endemic regions.
Basic and preclinical research: The team is developing recombinant antigens to improve disease diagnosis and to assess the risk of exposure to insects and ticks. We are also investigating the human immune response to pathogens transmitted by insects and ticks that transmit leishmaniasis and Lyme disease, respectively. In a study of leishmaniasis, we demonstrated the critical contribution of insect vectors in the initiation and establishment of disease. We demonstrated that insect-derived factors lead to acute inflammation key to pathogen establishment and dissemination in the host. The group also showed that bleeding at the site of insect or tick bites that is prolonged due to the anti-hemostatic effects of saliva triggers the cell stress response and heme oxygenase-1 induction, which dampens inflammation and promotes pathogen survival. These bite-specific immune events shape the early response to transmitted pathogens and have lasting effects on disease outcome. The group continues to investigate the host immune response to insect bites, which is essential to the development of therapeutics and novel interventions.
Clinical research: The team is currently developing a Controlled Human Infection Model (CHIM) of cutaneous leishmaniasis at the NIH clinical center in collaboration with Dr. Matthew Memoli (Director, LID Clinical Studies Unit) and Josh Lacsina (Assistant Clinical Investigator, Multiscale Systems Biology Section). The CHIM will be used to investigate the efficacy of promising vaccines and therapeutics against leishmaniasis using infected sand flies as a challenge. We are currently collaborating to validate a leading live attenuated vaccine developed by Hira Nakhasi (U.S. Food and Drug Administration), Abhay Satoskar (Ohio State University), and Greg Matlashewski (McGill University) that has shown high efficacy in preclinical studies.
Translational research: Dr. Kamhawi is collaborating with Dr. Naomi Aronson at the Uniformed Services University of the Health Sciences (USUHS) to investigate exposure to Leishmania parasites and sand fly vector bites in U.S. soldiers deployed to endemic areas of the Middle East. Leishmaniasis is also a chronic and fatal disease for dogs and has affected MWD deployed to endemic areas. Due to the chronicity of the disease, leishmaniasis-infected dogs can return to the U.S. while infected and can potentially transmit the parasites to local species of sand flies such as Lutzomyia (Lu.) shanonni and Lu. diabolica. Dr. Kamhawi is continuing to collaborate with Drs. Aronson and Davies at USUHS who are interested in investigating the prevalence of arthropod-borne pathogens in military facilities within the U.S. Additionally, in collaboration with Dr. Christy Peterson at Ohio State University (OSU), Dr. Kamhawi is investigating the transmissibility of Leishmania infections prevalent in foxhound dogs throughout the U.S. These studies are critical to assess the risk of emergence of leishmaniasis in the U.S. Dr. Kamhawi is also looking at drivers of transmission in cutaneous and visceral leishmaniasis foci and using diagnostic antigens her group developed as a surveillance tool to monitor direct human-vector contact in endemic populations. She is also working with Dr. Jesus Valenzuela to screen sera from inhabitants of Lyme disease and Rocky Mountain Spotted Fever endemic regions in the U.S. to develop diagnostic tools that assess exposure to ticks and tick-borne pathogens.
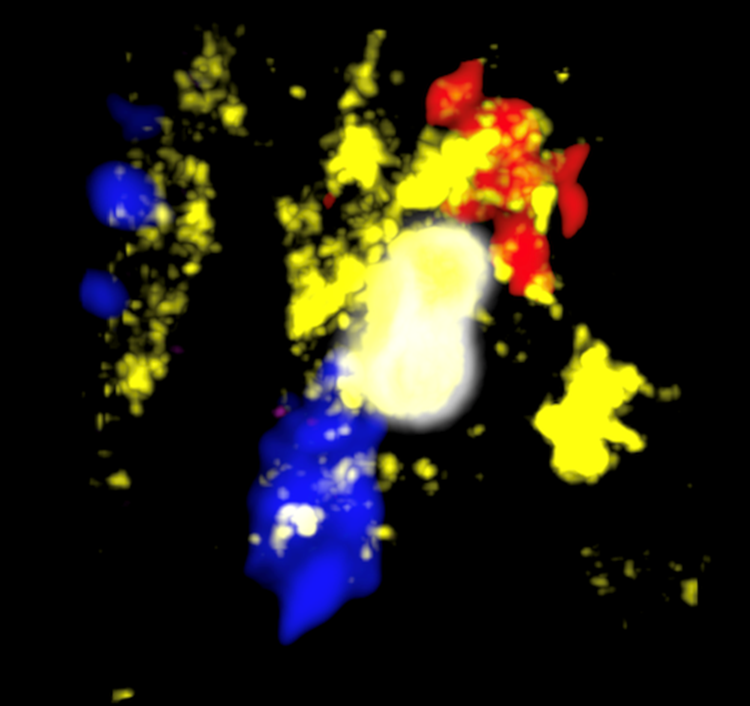
A tridimensional view of a macrophage (IBA-1, yellow; DAPI, blue) from mouse skin expressing heme oxygenase-1 (HO-1, red) after ingesting a red blood cell (TER-119, white) 18 hours after sand fly bites.
Biography
Education
Ph.D., Medical Entomology, Salford University, Salford, England
Languages Spoken
English, French, ArabicDr. Kamhawi received her Ph.D. in medical entomology from Salford University in England in 1990. She worked as an assistant professor at Yarmouk University in Jordan, where she worked for several years on leishmaniasis and hydatid disease, with a focus on disease transmission and risk factors in field settings. In 1997, she worked as a visiting scientist at NIAID’s Laboratory of Parasitic Diseases and became a staff scientist in 2000. In 2006, Dr. Kamhawi moved to NIAID’s Laboratory of Malaria and Vector Research (LMVR) as a core staff scientist and was awarded the honorary title of core associate scientist in 2014. In 2024, Dr. Kamhawi became a staff scientist two and is currently the group leader for insect-human-pathogen and insect-reservoir-pathogen interactions in the Vector Molecular Biology Section.
Clinical Studies
Selected Publications
Naomi E. Aronson, Fabiano Oliveira, Regis Gomes, William D. Porter, Robin S. Howard, Shaden Kamhawi and Jesus G. Valenzuela. Antibody Responses to Phlebotomus papatasi Saliva in American Soldiers With Cutaneous Leishmaniasis Versus Controls. Front. Trop. Dis, 2022.
Rupal M. Mody, Ines Elakhal-Naouar, Jeffrey E. Sherwood, Nancy L. Koles, Dutchabong Shaw, Daniel Bigley, Edgie Mark, Nathanial K. Copeland, Linda L. Jagodzinski, Rami M. Mukbel, Rebecca Smiley, Robert C. Duncan, Shaden Kamhawi, Selma M.B. Jeronimo, Robert F. DeFraites and Naomi E. Aronson. Asymptomatic Visceral Leishmania infantum Infection in U.S. Soldiers Deployed to Iraq. Clinical Infec Diseases, 2018.
Scorza BM, Mahachi KG, Cox AC, Toepp AJ, Leal-Lima A, Kumar Kushwaha A, Kelly P, Meneses C, Wilson G, Gibson-Corley KN, Bartholomay L, Kamhawi S, Petersen CA. Leishmania infantum xenodiagnosis from vertically infected dogs reveals significant skin tropism. PLoS Negl Trop Dis. 2021 Oct 6;15(10):e0009366.
Serafim TD, Iniguez E, Barletta ABF, Cecilio P, Doehl JSP, Short M, Lack J, Nair V, Disotuar M, Wilson T, Coutinho-Abreu IV, Meneses C, Andersen J, Alves E Silva TL, Oliveira F, Vega-Rodriguez J, Barillas-Mury C, Ribeiro JMC, Beverley SM, Kamhawi S, Valenzuela JG. Leishmania genetic exchange is mediated by IgM natural antibodies. Nature. 2023 Nov;623(7985):149-156.
Piyasiri SB, Senanayake S, Smaranayake N, Doh S, Iniguez E, Valenzuela JG, Kamhawi S, Karunaweera ND. Salivary antigens rPagSP02 and rPagSP06 are a reliable composite biomarker for evaluating exposure to Phlebotomus argentipes in Sri Lanka. Sci Rep. 2024 Oct 28;14(1):25863.
Iniguez E, Saha S, Petrellis G, Menenses C, Herbert S, Gonzalez-Rangel Y, Rowland T, Aronson NE, Rose C, Rafuse Haines L, Acosta-Serrano A, Serafim TD, Oliveira F, Srikantiah S, Bern C, Valenzuela JG, Kamhawi S. A Composite Recombinant Salivary Proteins Biomarker for Phlebotomus argentipes Provides a Surveillance Tool Postelimination of Visceral Leishmaniasis in India. J Infect Dis. 2022 Nov 11;226(10):1842-1851.
Patents
Fischer L, Kamhawi S, Valenzuela J, Suau HA, inventors; The United States of America as represented by the Secretary of the Department of Health and Human Services, assignee. Leishmania challenge model. United States patent US 8,906,358. 9 December 2014.
Valenzuela JG, Belkaid Y, Kamhawi S, Sacks D, Ribeiro JMC, inventors; The United States of America as represented by the Secretary of the Department of Health and Human Services, assignee. Anti-arthropod vector vaccines, methods of selecting and uses thereof. United States patent US 7,964,576. 21 June 2011.
Valenzuela JG, Ribeiro JMC, Kamhawi S, Belkaid Y, Fischer L, Audonnet JC, Milward F, inventors; The United States of America as represented by the Department of Health and Human Services, Merial Limited, assignees. P. ariasi polypeptides, p. perniciosuspolypeptides and methods of use. United States patent US 7,741,437. 22 Jun 2010.
Valenzuela JG, Belkaid Y, Kamhawi S, Sacks D, Ribeiro JMC, inventors; The United States of America as represented by the Department of Health and Human Services, assignee. Anti-arthropod vector vaccines, methods of selecting and uses thereof. United States patent US 7,388,089. 17 Jun 2008.
Visit the U.S. Patent and Trademark Office for a complete patent listing.
Participating Research Networks
- Uniformed Services University of the Health Sciences
- Ohio State University
- GHIT partnership with CBER, FDA; Ohio State University, McGill University, Gennova Biopharmaceuticals Limited, and Nagasaki University
- The University of Texas Medical Branch, Galveston
- University of California, San Francisco
- Oregon Health & Science University
- Yale School of Public Health
- Oswaldo Cruz Foundation in Rio de Janeiro

