Project Title: Analysis of Autoantibodies
NIAID Principal Investigator: Steve Holland, M.D.
Director, Division of Intramural Research, NIAID
Chief, Immunopathogenesis Section, LCIM


Project Title: Analysis of Autoantibodies
NIAID Principal Investigator: Steve Holland, M.D.
Director, Division of Intramural Research, NIAID
Chief, Immunopathogenesis Section, LCIM
Volunteer or get more information
Toll free: 1-800-411-1222
TTY: 1-866-411-1010
Se habla español
Email: prpl@mail.cc.nih.gov
Project Title: Analysis of intrapatient SARS-Cov-2 genetic variation
NIAID Principal Investigator: Eli Boritz, M.D., Ph.D
Chief, Virus Persistence and Dynamics Section, IL, VRC
TATFAR was created in 2009 to address the urgent threat of antimicrobial resistance (AMR). TATFAR’s technical experts from Canada, the European Union (EU), Norway, the United Kingdom, and the United States collaborate and share best practices to strengthen domestic and global efforts to combat AMR.
The Centers for Disease Control and Prevention (CDC) currently serves as the secretariat for TATFAR, providing administrative support and maintaining the website for the taskforce.
U.S. representatives to TATFAR include the U.S. Department of Health and Human Services (co-chair), NIH (NIAID), CDC, the Food and Drug Administration, the Biomedical Advanced Research and Development Authority, the U.S. Department of Agriculture, the U.S. Department of Defense, and the Environmental Protection Agency.
Read more about this network: TATFAR
TATFAR’s goal is to improve cooperation between the United States and EU in four key areas:
The Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) global partnership was created to help address the threat of antibiotic resistance.
CARB-X is funded by the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response (ASPR) in the United States Department of Health and Human Services; the Wellcome Trust in the United Kingdom; Germany’s Federal Ministry of Education and Research (BMBF); the United Kingdom Government’s Department of Health and Social Care (DHSC), through its Global Antimicrobial Resistance Innovation Fund (GAMRIF); the Bill & Melinda Gates Foundation; and the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health (NIH). For more information, please see Funding Partners.
Read more about this network: CARB-X
CARB-X is focused on the preclinical discovery and development and Phase 1 clinical trials of new antibacterial products (including therapeutics, vaccines, and diagnostics) to help address the threat of antibiotic resistance.
Over the last several decades there has been a continual withdrawal of pharmaceutical companies engaged in developing new antibiotics. In 1990, there were at least 18 large pharmaceutical companies actively developing antibiotics. As of April 2020, there are 4.
CARB-X provides funding for companies with innovative and promising solutions to antibiotic resistance. CARB-X also provides the business support and drug development expertise to that companies, including start-ups, need to increase their odds of success. NIAID provides technical support and preclinical drug development services to CARB-X awardees.
Anita Sheoran, Ph.D., Office of Biodefense, Research Resources and Translational Research
*This project is not currently being actively pursued.
Project Title: The role of the anti-commensal antibody repertoire in the differential outcomes of symptomatic versus asymptomatic infection in SARS-CoV-2
NIAID Principal Investigator: Yasmine Belkaid, Ph.D.
Former Chief, Metaorganism Immunity Section (LISB)

Dr. Saito received a D.V.M. from the State University of Londrina, Parana, Brazil. She completed a residency in clinical practice and small animal surgery as well as a Master of Science degree at the same University. Dr. Saito then earned her Ph.D. in veterinary medicine and infectious diseases at the State University of Sao Paulo, Brazil. In 2009, she moved to the U.S. to conduct postdoctoral research at the University of Texas Medical Branch at Galveston (UTMB). Later she was appointed as an Instructor at UTMB and as a Research Assistant Professor in 2017. Dr. Saito was recruited to the DIR NIAID in 2020 as a tenure-track investigator and is currently Chief of the Vector-Pathogen-Host Interaction Unit in the Laboratory of Bacteriology. She serves as a review editor for Frontiers in Cellular and Infection Microbiology and as ad hoc reviewer for numerous journals in her field. She is an advocate for diversity in the biomedical sciences.
Environmental changes are driving a global increase in arthropod borne infections that, in many cases, cause severe life-threatening illnesses. An example in the United States is human ehrlichioses, an emerging infectious disease caused by Ehrlichia spp. An increase in tick borne infections and recognition of new pathogen species in recent years emphasize the importance of understanding the disease initiation and determinants of susceptibility. In 2009, a new species of Ehrlichia was detected in human patients in United States, Ehrlichia muris eauclairensis, transmitted by the tick vector Ixodes scapularis. These blood-feeding arthropods use their salivary proteins to manipulate the host local immune reaction at the bite site, facilitating prolonged attachment. Many pathogens, before and during transmission, are known to modulate the tick tissue environment, as well as their own transcriptome/proteome. Therefore, the induction of infection in the skin involves an extremely complex interaction of modulatory factors from the tick, pathogen and the host immune response.
Our research group is interested in understanding the immune signaling involved in vector transmission of pathogens, primarily focusing on tissue-specific innate host responses induced by ehrlichial (and rickettsial) diseases. We believe that this initial response, at the skin site of the tick-pathogen interaction with the host, is associated with disease development and progression to severe outcome.
Our animal model of ehrlichiosis, using the natural mode of infection by tick transmission, results in increased bacterial replication and dissemination throughout the body and more severe outcome, compared to intradermal needle injection of ehrlichiae (Saito and Walker, 2015). The dermal reaction to tick inoculation of the ehrlichial pathogen demonstrates intense inflammatory infiltration and a strong IL-1 cytokine family signature. At the tick attachment site we observe intense tissue damage, with massive infiltration of granulocytes. The most impressive inflammatory infiltration is observed during the peak of tick feeding and after spontaneous detachment; however, we observed a dynamic response of the recruiting innate cells. Moreover, intradermal needle inoculation of bacteria did not induce extensive skin inflammation nor severe disease outcome. Similarly to other rickettsial infections, we identified a persistence of ehrlichial antigen at the skin of infected animals. These findings demonstrate induction of an inflammatory response, potentially associated with successful bacterial replication and disease pathogenesis.
Our ultimate goal is to better understand this complex vector-host-pathogen interaction at the cutaneous interface and its influence in immunopathogenesis of disease severity and/or development of protective immunity. By improving our knowledge of skin immune response to tick borne infections, we will be able to further evaluate the involvement of the initial immune-mediators on the development of dermal protective immunity to arthropod-transmitted diseases.
Liu Y, Zhou C, Su Z, Chang Q, Qiu Y, Bei J, Gaitas A, Xiao J, Drelich A, Khanipov K, Jin Y, Golovko G, Saito TB, Gong B. Endothelial Exosome Plays a Functional Role during Rickettsial Infection. mBio. 2021 May 11;12(3):e00769-21.
Saito TB, Bechelli J, Smalley C, Karim S, Walker DH. Vector Tick Transmission Model of Spotted Fever Rickettsiosis. Am J Pathol. 2019 Jan;189(1):115-123.
Smalley C, Bechelli J, Rockx-Brouwer D, Saito T, Azar SR, Ismail N, Walker DH, Fang R. Rickettsia australis Activates Inflammasome in Human and Murine Macrophages. PLoS One. 2016 Jun 30;11(6):e0157231.
Saito TB, Walker DH. A Tick Vector Transmission Model of Monocytotropic Ehrlichiosis. J Infect Dis. 2015 Sep 15;212(6):968-77.
Saito TB, Thirumalapura NR, Shelite TR, Rockx-Brouwer D, Popov VL, Walker DH. An animal model of a newly emerging human ehrlichiosis. J Infect Dis. 2015 Feb 1;211(3):452-61.
Shelite TR, Saito TB, Mendell NL, Gong B, Xu G, Soong L, Valbuena G, Bouyer DH, Walker DH. Hematogenously disseminated Orientia tsutsugamushi-infected murine model of scrub typhus [corrected]. PLoS Negl Trop Dis. 2014 Jul 10;8(7):e2966.
Environmental changes are driving a global increase in arthropod borne infections that, in many cases, cause severe life-threatening illnesses. An example in the United States is human ehrlichioses, an emerging infectious disease caused by Ehrlichia spp. An increase in tick borne infections and recognition of new pathogen species in recent years emphasize the importance of understanding the disease initiation and determinants of susceptibility. In 2009, a new species of Ehrlichia was detected in human patients in United States, Ehrlichia muris eauclairensis, transmitted by the tick vector Ixodes scapularis. These blood-feeding arthropods use their salivary proteins to manipulate the host local immune reaction at the bite site, facilitating prolonged attachment. Many pathogens, before and during transmission, are known to modulate the tick tissue environment, as well as their own transcriptome/proteome. Therefore, the induction of infection in the skin involves an extremely complex interaction of modulatory factors from the tick, pathogen and the host immune response.
Our research group is interested in understanding the immune signaling involved in vector transmission of pathogens, primarily focusing on tissue-specific innate host responses induced by ehrlichial (and rickettsial) diseases. We believe that this initial response, at the skin site of the tick-pathogen interaction with the host, is associated with disease development and progression to severe outcome.
Our animal model of ehrlichiosis, using the natural mode of infection by tick transmission, results in increased bacterial replication and dissemination throughout the body and more severe outcome, compared to intradermal needle injection of ehrlichiae (Saito and Walker, 2015). The dermal reaction to tick inoculation of the ehrlichial pathogen demonstrates intense inflammatory infiltration and a strong IL-1 cytokine family signature. At the tick attachment site we observe intense tissue damage, with massive infiltration of granulocytes. The most impressive inflammatory infiltration is observed during the peak of tick feeding and after spontaneous detachment; however, we observed a dynamic response of the recruiting innate cells. Moreover, intradermal needle inoculation of bacteria did not induce extensive skin inflammation nor severe disease outcome. Similarly to other rickettsial infections, we identified a persistence of ehrlichial antigen at the skin of infected animals. These findings demonstrate induction of an inflammatory response, potentially associated with successful bacterial replication and disease pathogenesis.
Our ultimate goal is to better understand this complex vector-host-pathogen interaction at the cutaneous interface and its influence in immunopathogenesis of disease severity and/or development of protective immunity. By improving our knowledge of skin immune response to tick borne infections, we will be able to further evaluate the involvement of the initial immune-mediators on the development of dermal protective immunity to arthropod-transmitted diseases.
Liu Y, Zhou C, Su Z, Chang Q, Qiu Y, Bei J, Gaitas A, Xiao J, Drelich A, Khanipov K, Jin Y, Golovko G, Saito TB, Gong B. Endothelial Exosome Plays a Functional Role during Rickettsial Infection. mBio. 2021 May 11;12(3):e00769-21.
Saito TB, Bechelli J, Smalley C, Karim S, Walker DH. Vector Tick Transmission Model of Spotted Fever Rickettsiosis. Am J Pathol. 2019 Jan;189(1):115-123.
Smalley C, Bechelli J, Rockx-Brouwer D, Saito T, Azar SR, Ismail N, Walker DH, Fang R. Rickettsia australis Activates Inflammasome in Human and Murine Macrophages. PLoS One. 2016 Jun 30;11(6):e0157231.
Saito TB, Walker DH. A Tick Vector Transmission Model of Monocytotropic Ehrlichiosis. J Infect Dis. 2015 Sep 15;212(6):968-77.
Saito TB, Thirumalapura NR, Shelite TR, Rockx-Brouwer D, Popov VL, Walker DH. An animal model of a newly emerging human ehrlichiosis. J Infect Dis. 2015 Feb 1;211(3):452-61.
Shelite TR, Saito TB, Mendell NL, Gong B, Xu G, Soong L, Valbuena G, Bouyer DH, Walker DH. Hematogenously disseminated Orientia tsutsugamushi-infected murine model of scrub typhus [corrected]. PLoS Negl Trop Dis. 2014 Jul 10;8(7):e2966.

Human monoclonal antibodies are emerging as powerful tools in combating infectious disease, both as direct prophylactics and as reagents to identify vulnerable sites on pathogens to guide vaccine design. At the Antibody Biology Unit (ABU), we aim to use cutting-edge technology to study B cells at the single cell level and to identify and characterize human monoclonal antibodies against a range of pathogens. We have two major aims:
The primary focus of the unit will be on malaria. Plasmodium falciparum causes approximately 400,000 deaths a year and remains a serious global health threat. Antibodies have been shown to be key mediators of protection against different stages of the P. falciparum life cycle, but the antibody response to malaria has only recently been studied at high resolution. The biology of the antibody response to P. falciparum is complex and fascinating. Recently, we identified broadly reactive antibodies from individuals living in malaria-endemic areas that contain a LAIR1 insert (an extra immunoglobulin-like domain) that is originally encoded in a different chromosome. This insert confers broad reactivity and is somatically mutated along with the rest of the antibody. This insertion event appears to be quite common in individuals living in different malaria-endemic regions (5-10% of individuals). In a separate study, we identified potent human monoclonal antibodies targeting a novel epitope on the P. falciparum circumsporozoite protein, the major sporozoite coat protein. This site is now being investigated as a new vaccine candidate.
Our platform is adaptable to any target. This unit will also study human monoclonal antibodies against other infectious agents, including Mycobacterium tuberculosis and SARS-CoV-2, as well as non-infectious targets.
Joshua Tan, Ph.D., is a Stadtman Tenure-track Investigator and an NIH Distinguished Scholar in the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases. He received his Ph.D. from the University of Oxford, England. Prior to joining the NIH, he was awarded the Pfizer Research Prize for his malaria work and the Sir Henry Wellcome Postdoctoral Fellowship to investigate human monoclonal antibodies that target the malaria-causing parasite P. falciparum.
Andrew Cooper, Ph.D., Postdoctoral Fellow
Cherrelle Dacon, Ph.D., Postdoctoral Fellow
Divya Mohan, Biologist
Lauren Purser, Lab Manager
Lawrence Wang, Visiting PhD Student
Courtney Tucker, Ph.D. Student
Cho H, Gonzales-Wartz KK, Huang D, Yuan M, Peterson M, Liang J, Beutler N, Torres JL, Cong Y, Postnikova E, Bangaru S, Talana CA, Shi W, Yang ES, Zhang Y, Leung K, Wang L, Peng L, Skinner J, Li S, Wu NC, Liu H, Dacon C, Moyer T, Cohen M, Zhao M, Lee FE, Weinberg RS, Douagi I, Gross R, Schmaljohn C, Pegu A, Mascola JR, Holbrook M, Nemazee D, Rogers TF, Ward AB, Wilson IA, Crompton PD and Tan J. 2021 Bispecific antibodies targeting distinct regions of the spike protein potently neutralize SARS-CoV-2 variants of concern. Sci Transl Med 13, eabj5413.
Tan J, Cho H, Pholcharee T, Pereira LS, Doumbo S, Doumtabe D, Flynn BJ, Schön A, Kanatani S, Aylor SO, Oyen D, Vistein R, Wang L, Dillon M, Skinner J, Peterson M, Li S, Idris AH, Molina-Cruz A, Zhao M, Olano LR, Lee PJ, Roth A, Sinnis P, Barillas-Mury C, Kayentao K, Ongoiba A, Francica JR, Traore B, Wilson IA, Seder RA and Crompton PD. 2021. Functional human IgA targets a conserved site on malaria sporozoites. Sci Transl Med 13, abg2344.
Tan J, Piccoli L and Lanzavecchia A. 2019. The antibody response to Plasmodium falciparum: cues for vaccine design and the discovery of receptor-based antibodies. Annu Rev Immunol 37, 225-246.
Tan J, Sack BK, Oyen D, Zenklusen I, Piccoli L, Barbieri S, Foglierini M, Fregni CS, Marcandalli J, Jongo S, Abdulla S, Perez L, Corradin G, Varani L, Sallusto F, Sim BKL, Hoffman SL, Kappe SHI, Daubenberger C, Wilson IA and Lanzavecchia A. 2018. A public antibody lineage that potently inhibits malaria infection through dual binding to the circumsporozoite protein. Nat Med 24, 401-407.
Pieper K, Tan J, Piccoli L, Foglierini M, Barbieri S, Chen Y, Fregni CS, Wolf T, Jarrossay D, Anderle M, Abdi A, Ndungu FM, Doumbo OK, Traore B, Tran TM, Jongo S, Zenklusen I, Crompton PD, Daubenberger C, Bull PC, Sallusto F and Lanzavecchia A. 2017. Public antibodies to malaria antigens generated by two LAIR1 insertion modalities. Nature 548, 597-601.
Tan J, Pieper K, Piccoli L, Abdi A, Foglierini M, Geiger R, Tully CM, Jarrossay D, Ndungu FM, Wambua J, Bejon P, Fregni CS, Fernandez-Rodriguez B, Barbieri S, Bianchi S, Marsh K, Thathy V, Corti D, Sallusto F, Bull P and Lanzavecchia A. 2016. A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens. Nature 529, 105-109.
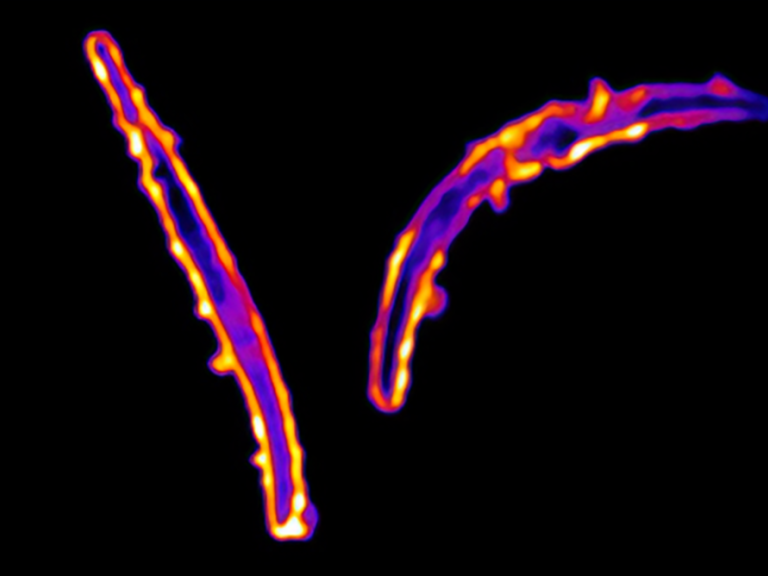
January 3, 2025
New antibodies that bind to a previously untargeted portion of the malaria parasite could lead to new monoclonal antibody treatments and vaccines for malaria.
Human monoclonal antibodies are emerging as powerful tools to combat infectious disease. At the Antibody Biology Unit (ABU), we aim to use cutting-edge technology to study B cells at the single-cell level and to identify and characterize human monoclonal antibodies against a range of pathogens. The Unit currently focuses on diseases with substantial burden globally or in the United States.
ABU has two main objectives:
Dacon C, Moskovitz R, Swearingen K, Da Silva Pereira L, Flores-Garcia Y, Aleshnick M, Kanatani S, Flynn B, Molina-Cruz A, Wollenberg K, Traver M, Kirtley P, Purser L, Dillon M, Bonilla B, Franco A, Petros S, Kritzberg J, Tucker C, Paez GG, Gupta P, Shears MJ, Pazzi J, Edgar JM, Teng AA, Belmonte A, Oda K, Doumbo S, Krymskaya L, Skinner J, Li S, Ghosal S, Kayentao K, Ongoiba A, Vaughan A, Campo JJ, Traore B, Barillas-Mury C, Wijayalath W, Idris A, Crompton PD, Sinnis P, Wilder BK, Zavala F, Seder RA, Wilson IA, Tan J. Protective antibodies target cryptic epitope unmasked by cleavage of malaria sporozoite protein. Science. 2025 Jan 3;387(6729):eadr0510.
Wang LT, Cooper AJR, Farrell B, Miura K, Diouf A, Müller-Sienerth N, Crosnier C, Purser L, Kirtley PJ, Maciuszek M, Barrett JR, McHugh K, Ogwang R, Tucker C, Li S, Doumbo S, Doumtabe D, Pyo CW, Skinner J, Nielsen CM, Silk SE, Kayentao K, Ongoiba A, Zhao M, Nguyen DC, Lee FE, Minassian AM, Geraghty DE, Traore B, Seder RA, Wilder BK, Crompton PD, Wright GJ, Long CA, Draper SJ, Higgins MK, Tan J. Natural malaria infection elicits rare but potent neutralizing antibodies to the blood-stage antigen RH5. Cell. 2024 Sep 5;187(18):4981-4995.e14.
Dacon C, Peng L, Lin TH, Tucker C, Lee CD, Cong Y, Wang L, Purser L, Cooper AJR, Williams JK, Pyo CW, Yuan M, Kosik I, Hu Z, Zhao M, Mohan D, Peterson M, Skinner J, Dixit S, Kollins E, Huzella L, Perry D, Byrum R, Lembirik S, Murphy M, Zhang Y, Yang ES, Chen M, Leung K, Weinberg RS, Pegu A, Geraghty DE, Davidson E, Doranz BJ, Douagi I, Moir S, Yewdell JW, Schmaljohn C, Crompton PD, Mascola JR, Holbrook MR, Nemazee D, Wilson IA, Tan J. Rare, convergent antibodies targeting the stem helix broadly neutralize diverse betacoronaviruses. Cell Host Microbe. 2023 Jun 14;31(6):1071-1072.
Dacon C, Tucker C, Peng L, Lee CD, Lin TH, Yuan M, Cong Y, Wang L, Purser L, Williams JK, Pyo CW, Kosik I, Hu Z, Zhao M, Mohan D, Cooper AJR, Peterson M, Skinner J, Dixit S, Kollins E, Huzella L, Perry D, Byrum R, Lembirik S, Drawbaugh D, Eaton B, Zhang Y, Yang ES, Chen M, Leung K, Weinberg RS, Pegu A, Geraghty DE, Davidson E, Douagi I, Moir S, Yewdell JW, Schmaljohn C, Crompton PD, Holbrook MR, Nemazee D, Mascola JR, Wilson IA, Tan J. Broadly neutralizing antibodies target the coronavirus fusion peptide. Science. 2022 Jul 12:eabq3773.
Cho H, Gonzales-Wartz KK, Huang D, Yuan M, Peterson M, Liang J, Beutler N, Torres JL, Cong Y, Postnikova E, Bangaru S, Talana CA, Shi W, Yang ES, Zhang Y, Leung K, Wang L, Peng L, Skinner J, Li S, Wu NC, Liu H, Dacon C, Moyer T, Cohen M, Zhao M, Lee FE, Weinberg RS, Douagi I, Gross R, Schmaljohn C, Pegu A, Mascola JR, Holbrook M, Nemazee D, Rogers TF, Ward AB, Wilson IA, Crompton PD, Tan J. Bispecific antibodies targeting distinct regions of the spike protein potently neutralize SARS-CoV-2 variants of concern. Sci Transl Med. 2021 Oct 20;13(616):eabj5413.
Tan J, Pieper K, Piccoli L, Abdi A, Perez MF, Geiger R, Tully CM, Jarrossay D, Maina Ndungu F, Wambua J, Bejon P, Fregni CS, Fernandez-Rodriguez B, Barbieri S, Bianchi S, Marsh K, Thathy V, Corti D, Sallusto F, Bull P, Lanzavecchia A. A LAIR1 insertion generates broadly reactive antibodies against malaria variant antigens. Nature. 2016 Jan 7;529(7584):105-109.
*This project is not currently being actively pursued.
Brescia Civil Hospital
Brescia, Italy
Principal Investigators: Luisa Imberti and Eugenia Quiros
Children's Hospital of Philadelphia
Philadelphia, Pennsylvania, USA
Principal Investigator: Sarah Henrickson