Infectious Disease Pathogenesis Section
Established in 2003
Derron A. Alves, D.V.M., Diplomate ACVP
Chief, Infectious Disease Pathogenesis Section
Specialty(s): Infectious Disease

Major Areas of Research
- Infectious disease pathogenesis
- Animal model development and support
- Laboratory animal pathology and special studies
Program Description
The Infectious Disease Pathogenesis Section (IDPS), utilizing a collaborative and integrative One-Health approach, directly supports NIAID investigators, programs, and collaborators through the incorporation of pathology-based, animal model development and use, and IACUC-approved research in order to facilitate and improve diagnoses, treatments, preventions, and medical countermeasures of infectious diseases in humans. The IDPS conducts comprehensive postmortem examination (i.e., necropsy) and tissue collection training on laboratory animal species; and provides complete microscopic tissue evaluation utilizing a wide array of diagnostic, molecular, and special studies to support spontaneous and experimental disease pathogenesis research primarily involving significant and/or emerging public health threats. Equipped with and working alongside other core services, our board-certified veterinary pathologist(s) and highly specialized technical team is committed to providing concise and dependable results to collaborating researchers as well as publication-worthy summary findings (to include photomicrographs) in order to continue to advance the missions and vision of the NIAID.
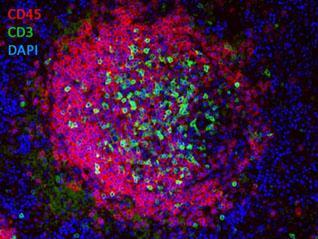
Double-Label Immunofluorescence of a Lymphoid Follicle Left: CD45R/B220-labeled B-lymphocytes (red), CD3-labeled T-lymphocytes (green), and DAPI (blue).
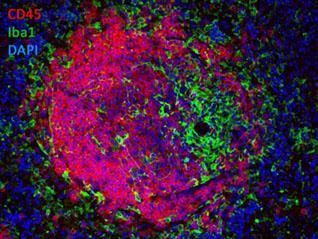
Double-Label Immunofluorescence of a Lymphoid Follicle Right: CD45R/B220-labeled B-lymphocytes (red), Iba1-labeled macrophages (green), and DAPI (blue)
Biography
Education
D.V.M., North Carolina State University College of Veterinary Medicine
B.S., Tuskegee University
Dr. Derron A.(“Tony”) Alves joined the IDPS in 2022 after 23 years of active-duty service in the U.S. Army, culminating his career as the U.S. Army Surgeon General’s Consultant for Veterinary Pathology and Deputy Director, Veterinary Services and Public Health Sanitation Directorate, Army Public Health Center, Aberdeen Proving Ground, MD. Dr. Alves earned a B.S. (Animal and Poultry Science) from Tuskegee University and was commissioned through ROTC in the U.S. Army Medical Service Corps. He earned a Doctor of Veterinary Medicine (DVM) degree from the North Carolina State University College of Veterinary Medicine. Following a three-year post-doctoral veterinary pathology residency at the Armed Forces Institute of Pathology, Washington, D.C, he was board certified (Diplomate) by the American College of Veterinary Pathologists in 2006. Post residency, Dr. Alves was assigned as a biodefense research pathologist at the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID), Fort Detrick, MD followed by multiple assignments to include the Director, DoD Veterinary Pathology Residency (DODVPR) Program, Joint Pathology Center (JPC), Defense Health Agency (DHA), in Silver Spring, MD. Select military decorations and awards include the Legion of Merit (2), Bronze Star Medal, Order of Military Medical Merit, and the Army Surgeon General’s distinguished “A” Proficiency Designation for veterinary pathology. Dr. Alves is a USDA-certified Foreign Animal Disease Diagnostician (FADD), and a distinguished research pathologist with numerous first- or co-authored manuscripts in peer reviewed scientific journals and book chapters, some involving agents of national security interests at Biosafety Levels 3 and 4.
Selected Publications
Frick OM, Livingston VA, Whitehouse CA, Norris SL, Alves DA, Facemire PR, Reed DS, Nalca A. The Natural History of Aerosolized Francisella tularensis Infection in Cynomolgus Macaques. Pathogens. 2021 May 13;10(5):597.
Dubey JP, Ferreira LR, Alsaad M, Verma SK, Alves DA, Holland GN, McConkey GA. Experimental Toxoplasmosis in Rats Induced Orally with Eleven Strains of Toxoplasma gondii of Seven Genotypes: Tissue Tropism, Tissue Cyst Size, Neural Lesions, Tissue Cyst Rupture without Reactivation, and Ocular Lesions. PLoS One. 2016 May 26;11(5):e0156255.
Alves DA, Honko AN, Kortepeter MG, Sun M, Johnson JC, Lugo-Roman LA, Hensley LE. Necrotizing Scleritis, Conjunctivitis, and Other Pathologic Findings in the Left Eye and Brain of an Ebola Virus-Infected Rhesus Macaque (Macaca mulatta) With Apparent Recovery and a Delayed Time of Death. J Infect Dis. 2016 Jan 1;213(1):57-60.
Glynn AR, Alves DA, Frick O, Erwin-Cohen R, Porter A, Norris S, Waag D, Nalca A. Comparison of experimental respiratory tularemia in three nonhuman primate species. Comp Immunol Microbiol Infect Dis. 2015 Apr;39:13-24.
Hensley LE, Alves DA, Geisbert JB, Fritz EA, Reed C, Larsen T, Geisbert TW. Pathogenesis of Marburg hemorrhagic fever in cynomolgus macaques. J Infect Dis. 2011 Nov;204 Suppl 3:S1021-31.
Alves DA, Glynn AR, Steele KE, Lackemeyer MG, Garza NL, Buck JG, Mech C, Reed DS. Aerosol exposure to the angola strain of marburg virus causes lethal viral hemorrhagic Fever in cynomolgus macaques. Vet Pathol. 2010 Sep;47(5):831-51.
Services Offered
- Core Service led by a veterinary pathologist board certified by the American College of Veterinary Pathologists (ACVP) and consisting of expertly trained histotechnologists
- Pre-protocol/study consultation for animal model studies requiring all aspects of pathology support
- Conduct postmortem examinations in laboratory animals of a wide variety of species
- Interpret and document (written and photographic) gross pathologic findings
- Tissue trimming and processing, paraffin embedding, and slide sectioning following postmortem and/or antemortem tissue (e.g., biopsy) submission
- Conduct routine histopathologic (and histochemical) tissue examination and photomicroscopy
- Special histochemical staining and interpretation for infectious and non-infectious agents as well as cellular/tissue components utilizing, but not limited to, gram stains, PAS, GMS, Ziehl-Neelsen, Perl’s Prussian blue, Masson’s Trichrome, etc
- Conduct and interpret immunohistochemical (IHC), immunofluorescence (IF) and in situ hybridization (ISH) findings on formalin-fixed paraffin-embedded tissue sections
- Protocol development for new antibodies, including antigen retrieval; as well as utilize commercially available antibodies optimized for use in rodents and nonhuman primates
- Utilize and collaborate with other core services (e.g., electron microscopy, confocal microscopy) in order to enhance research support
- Interpret clinical pathology data (limited)
- Generate timely interim findings and present data to investigators in a concise, dependable manner throughout the course of a study
- Produce final summary pathology reports for scientific peer reviewed manuscript preparation, publication, and/or oral platform presentation
Location
Twinbrook III, 2nd Floor
Office: Room 2W-01
Lab: Room 2W-03
Research Group
The IDPS conducts comprehensive postmortem examination (i.e., necropsy) and tissue collection training on laboratory animal species; and provides complete microscopic tissue evaluation utilizing a wide array of diagnostic, molecular, and special studies to support spontaneous and experimental disease pathogenesis research primarily involving significant and/or emerging public health threats.


