NIAID supports clinical research trials to develop better ways of diagnosing, treating, and preventing Lyme disease. People who have been diagnosed with Lyme disease or who suspect that they have Lyme disease may be eligible to participate.


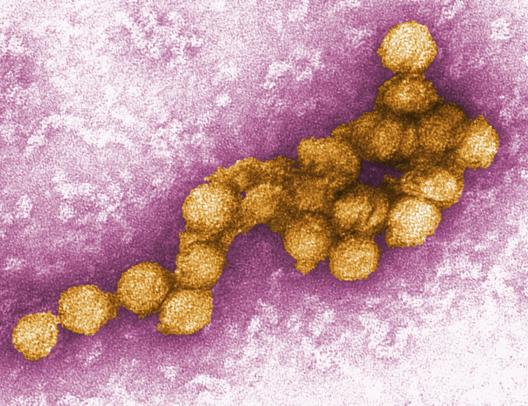
West Nile virus (WNV) is a member of the flavivirus family, which includes other mosquito-borne viruses such as dengue and Zika. WNV emerged for the first time in the Western Hemisphere in New York City in 1999 and has since spread across the United States. It is now the most common arthropod-borne virus found in the U.S.
Most people (about 8 out of 10) who are infected with WNV develop no symptoms at all, while a smaller proportion develop mild symptoms of fever, body aches, skin rash, and swollen lymph nodes. If the virus crosses the blood-brain barrier, however, it can cause life-threatening conditions that include inflammation of the brain and spinal cord. There are no specific therapies for serious WNV infection and there is currently no vaccine available to prevent infection.
NIAID supports research aimed at better understanding of the interactions between WNV virus, mosquitoes, and the human immune response, as well as broad-spectrum anti-flavivirus drug discovery efforts.
To learn about risk factors for WNV and current prevention and treatment strategies visit the MedlinePlus West Nile virus site.
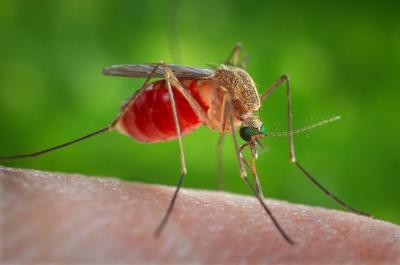
This is an enlarged view of a Culex quinquefasciatus mosquito that had landed upon the skin of a human host, and was about to insert its needle-sharp proboscis through the skin, which would enable it to obtain its blood meal.
Lyme disease, or borreliosis, is caused by the bacterium Borrelia burgdorferi and is transmitted to humans through the bite of an infected blacklegged deer tick. It is the most common tickborne infectious disease in the United States.
State health departments reported 42,743 confirmed or probable cases to the Centers for Disease Control and Prevention (CDC) in 2017. Reported cases are not believed to reflect the actual incidence of Lyme disease, and CDC estimates that 300,000 cases likely occur annually. The incidence of Lyme disease, as with many other tick-borne diseases, has increased dramatically over the past 10 years.
To learn about risk factors for Lyme Disease and current prevention and treatment strategies visit the MedlinePlus Lyme Disease site.
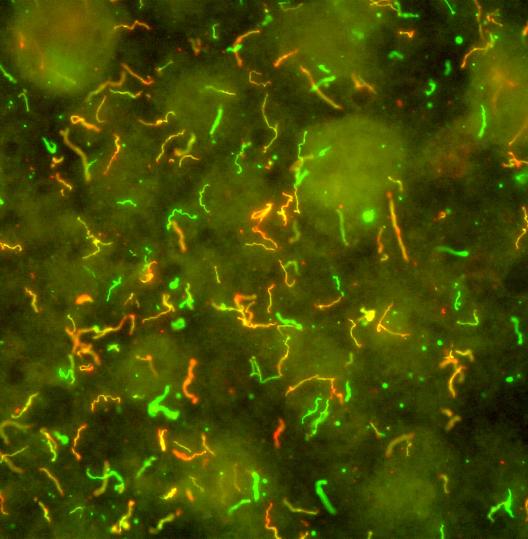
NIAID supports clinical research trials to develop better ways of diagnosing, treating, and preventing Lyme disease. People who have been diagnosed with Lyme disease or who suspect that they have Lyme disease may be eligible to participate.
Scientists are searching for better ways to diagnose, treat, and prevent tickborne diseases. They are also looking for ways to control the tick populations that transmit microbes.

The overarching goals of the NIAID Lyme disease research program are to develop better
methods of diagnosing, treating, and preventing this disease in humans. To accomplish these
objectives, the NIAID Lyme disease research portfolio includes a broad range of activities
focusing on the pathogen, the vector, and the vertebrate hosts.
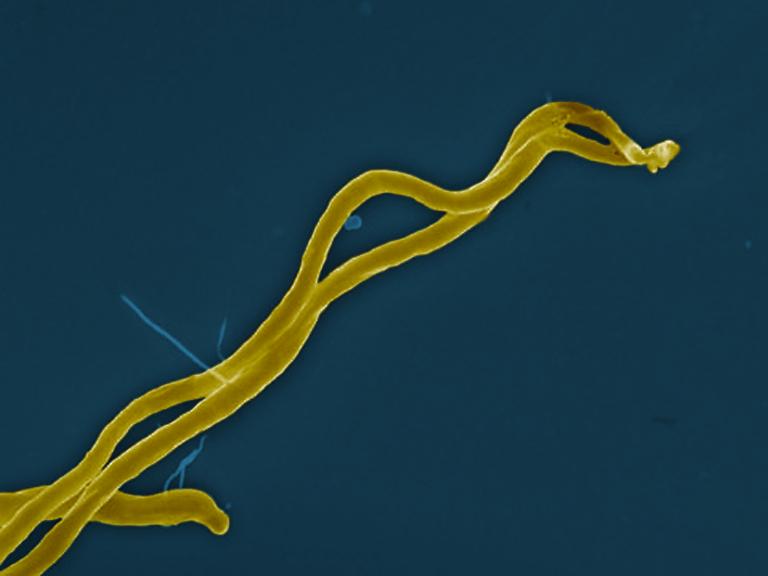
NIAID scientists and colleagues are investigating a potential treatment strategy against Lyme disease that would directly suppress Borrelia burgdorferi, the bacterium that causes the disease.