
A research study testing an investigational COVID-19 treatment called anti-coronavirus hyperimmune intravenous immunoglobulin (hIVIG).
What Does the Study Involve?
The Outpatient Treatment with Anti-Coronavirus Immunoglobulin (OTAC) study is testing hIVIG as a treatment for COVID-19.
What is hIVIG? hIVIG stands for “hyperimmune intravenous immunoglobulin.” hIVIG is made from blood plasma donated by healthy people who have recovered from COVID-19 and have been vaccinated against COVID-19. Plasma is the liquid part of your blood.
The plasma is collected and purified to make hIVIG. It contains high levels of antibodies that fight the virus that causes COVID-19 (SARS-CoV-2). Antibodies are natural proteins made by the body to fight or prevent infection.
The OTAC study is testing hIVIG to answer the following questions:
- Can this investigational treatment help people have fewer COVID-19 symptoms?
- Can this investigational treatment help people with COVID-19 stay out of the hospital?
- Is this investigational treatment safe for people with COVID-19 who may have a higher risk of getting very sick from their infection?
If you join this study, you will receive one dose of either hIVIG or a placebo (salt water which has no drug in it). Your chance of receiving hIVIG will be 50%, which is just like flipping a coin. You will get hIVIG or placebo as a drip or infusion (IV) into one of your veins. The study team will watch you while the infusion is being given and for some time after. You will get the usual standard care for COVID-19 that you would receive even if you were not in a study. Your decision to join the study is purely up to you, and you can change your mind at any time.
How hIVIG Production and Administration Work
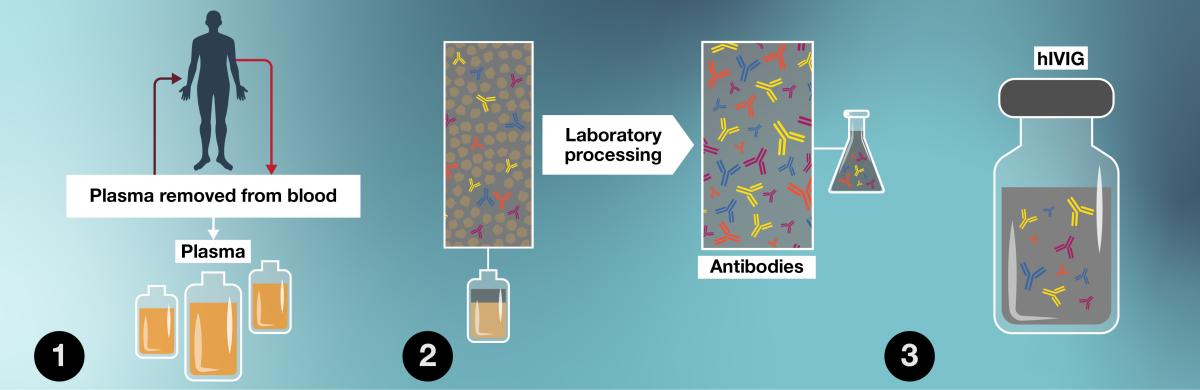
- Plasma is removed from blood donated by healthy people who have recovered from COVID-19 and been vaccinated against COVID-19.
- Antibodies are separated from the plasma in a lab and germs are destroyed.
- Antibodies from many people are combined to make hIVIG.
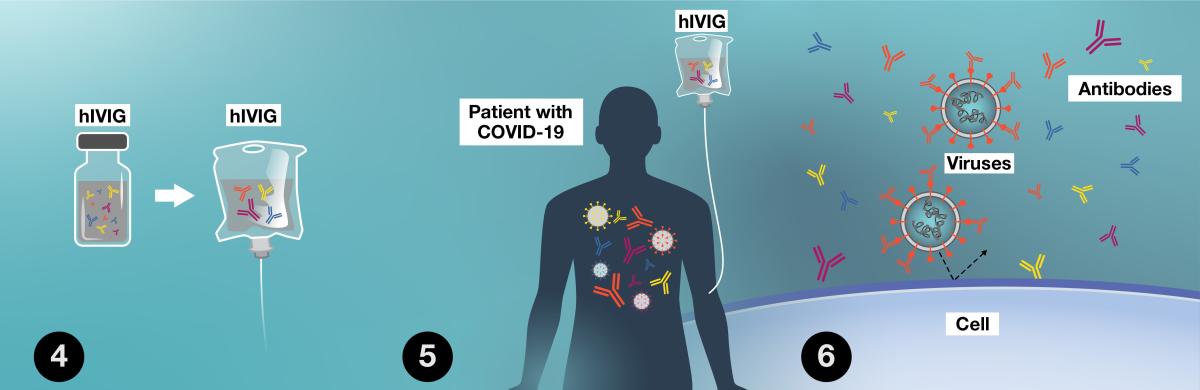
- hIVIG has high concentration of antibodies against SARS CoV-2, the virus that causes COVID-19.
- hIVIG is given as a single infusion into a vein (blood vessel).
- hIVIG antibodies may bind to SARS-CoV-2 and may stop it from entering your cells. This may keep you from getting very ill.
Who Can Participate?
If you recently tested positive for COVID-19, this study may be for you. Clinical trials testing investigational COVID-19 treatments need people from diverse backgrounds. This will help researchers find treatments that work for more people. Each person who takes part in the OTAC study will meet specific conditions, including:
- Age – At least 55 years old OR at least 18 years old with a weakened immune system or on medication that suppresses the immune system
- COVID-19 Status – Not currently in the hospital, and tested positive for COVID-19 within the past 5 days
- Symptoms – No symptoms OR COVID-19 symptoms for no longer than 5 days
There are other requirements that the study team will explain to you. If you do not meet the requirements, or if there is not a study site near you, there may be other COVID-19 studies that you can participate in.
Where Is It Taking Place?
Visit ClincialTrials.gov to find an OTAC study site near you.
Number of Visits Required
Your participation in this study will last for 28 days. You will check your temperature and your oxygen levels at home. Your study team will give you easy-to-use equipment at no cost to you to do this. Your study team will check on your health the day after the infusion and then four more times after that. Most of the health checks can be done over the phone.
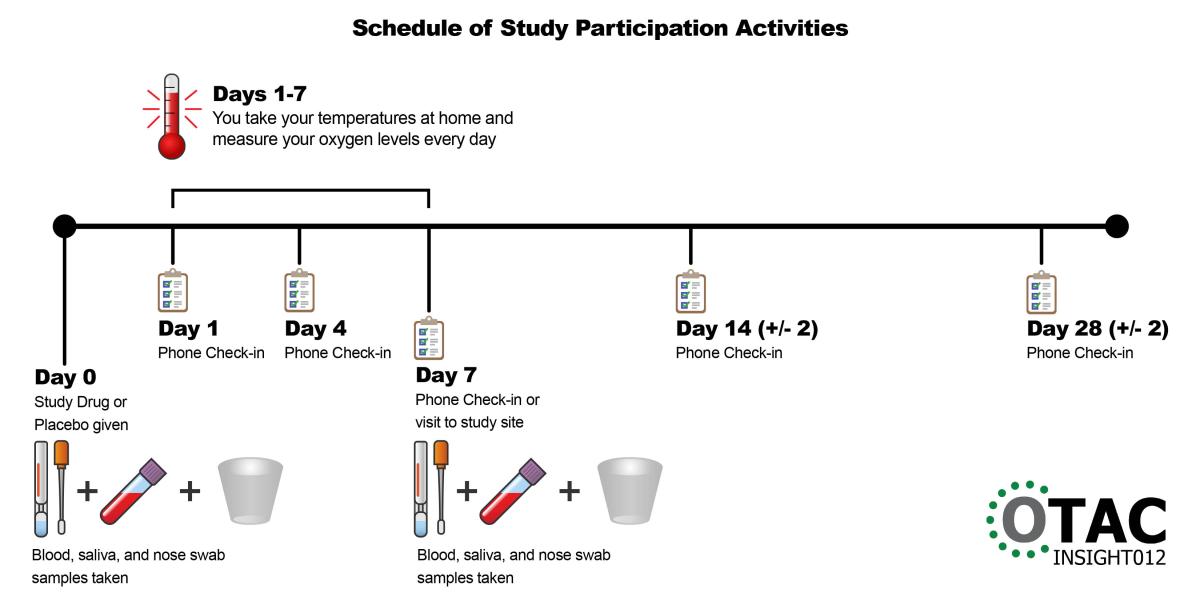
- Day 0 – Study drug or placebo given; blood, saliva, and nose swab samples taken
- Days 1-7 – You take your temperatures at home and measure your oxygen levels every day
- Day 1 – Phone check-in
- Day 4 – Phone check-in
- Day 7 – Phone check-in and home visit by health care provider, or visit to study site; blood, saliva, and nose swab samples taken
- Day 14 (+/-2) – Phone check-in
- Day 28 (+/-2) – Phone check-in
Is There a Cost?
There is no cost to participate in this study.
Is Compensation Provided?
Compensation for each participant will be based on the study visits they make and the samples collected from them.
Steps To Participate and Contact Information
If you are interested in joining this study or you would like more information, please visit ClincialTrials.gov to find a nearby OTAC study location and send an email to the email address shown for the nearest study location, or to the contact listed for the University of Minnesota. For further information about this OTAC study or to ask about joining the study, you may also send an email to Combat COVID Help.
Resources
Study Information Downloadable Presentation

Answers to Other Questions You Might Have
What Are Clinical Research Studies?
A clinical research study helps experts find treatments that are safe and effective. It is important that these studies include people from diverse backgrounds. This helps researchers develop and test new treatments that will work for more people.
What Is a Placebo?
A placebo looks like the study drug but does not have any active drugs in it. Placebos should not make someone feel better or worse. Researchers compare how people react to both placebos and study drugs to learn if the study drug is safe and helps to treat the disease being studied.
Can I Change My Mind After I Join the Study?
Yes. Taking part in this research study is voluntary; it is your choice. You can change your mind at any time and leave the study. Leaving the study will not affect the medical care you receive.

