Combination antiretroviral therapy directly targets HIV and can keep levels of the virus low in the blood, resulting in far better health outcomes for those who take these lifesaving medications. However, sometimes other conditions and complications associated with HIV infection can warrant further intervention. NIAID and other institutes at NIH support research that works toward ensuring the full health of people living with HIV.
Developing Treatments for HIV Co-Infections
Many people living with HIV acquire co-infections because of vulnerabilities in their immune systems and shared risk factors for HIV and other diseases, like illicit drug use or living in an area with a high prevalence of certain pathogens. These infections can occur even in those whose HIV is well-treated and who have strong immune function relative to people living with advanced HIV, or AIDS. The most serious infections that commonly occur alongside HIV both in the United States and around the world are viral hepatitis, including hepatitis C and hepatitis B, and tuberculosis, or tuberculosis.
As many as 5 million people living with HIV around the world also have hepatitis C virus, or HCV. HCV can be a chronic condition that can lead to life-threatening liver failure and liver cancer. Traditional interferon-based treatments have not worked as well in people also living with HIV. However, researchers led by NIAID and Gilead Sciences have developed new therapies that can cure even complicated cases of HCV without serious side effects.
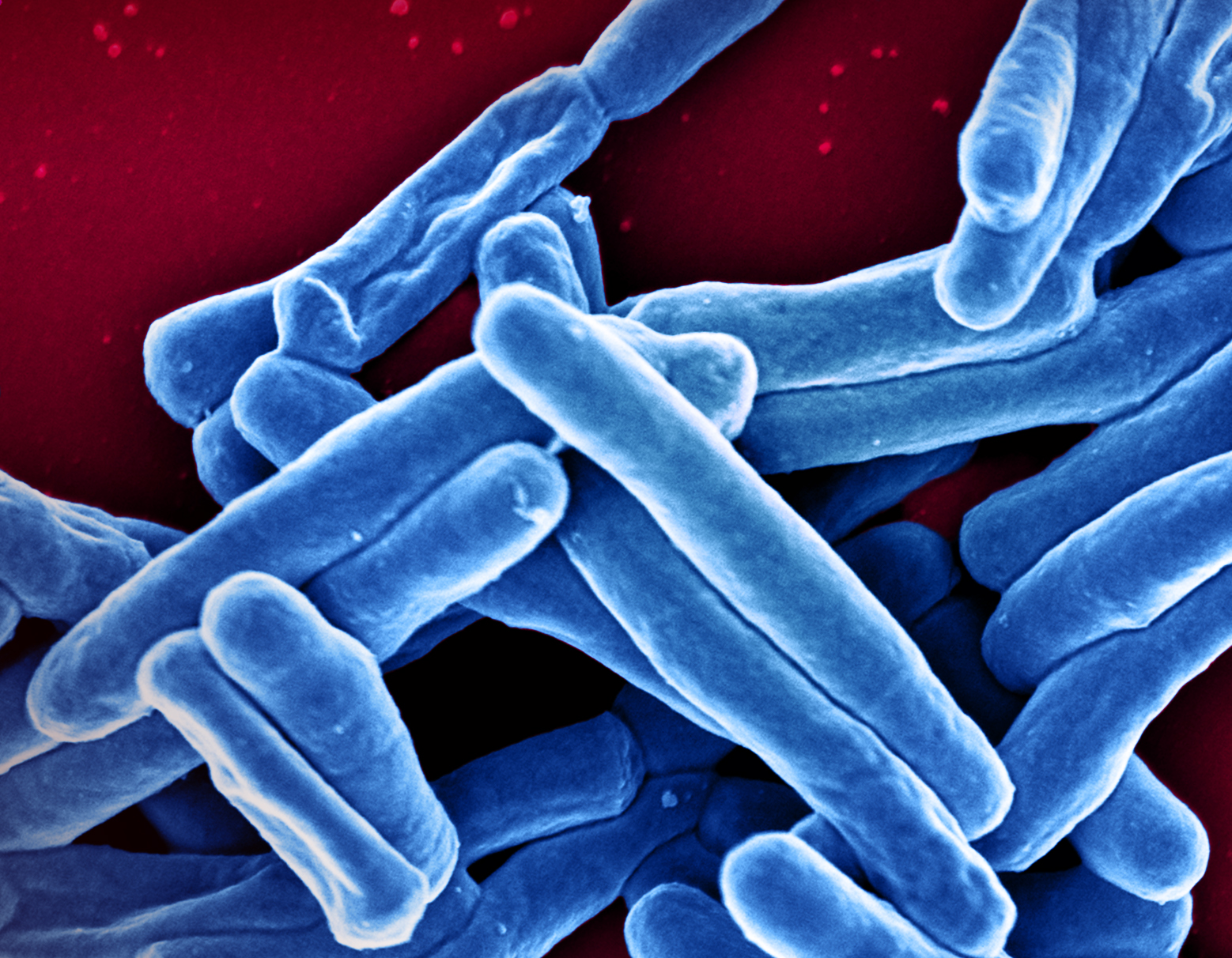
People living with HIV are also at an increased risk of developing tuberculosis, or TB, a disease caused by the bacteria Mycobacterium tuberculosis that usually begins in the lungs. This increased risk can be as much as 26 to 31 times higher than someone without HIV, according to the World Health Organization and appears to persist even when HIV is well-controlled with antiretroviral therapy (ART). TB is the leading cause of death among people living with HIV worldwide, with most cases occurring in developing countries. Additionally, having HIV can make TB more difficult to diagnose, and medications used to treat both infections may interact negatively. In addition to efforts to address the burden of TB disease acting alone, NIAID supports research to improve TB prevention, diagnosis, and treatment in the context of HIV infection.
To that end, in 2018, NIAID-supported researchers found that a one-month antibiotic regimen to prevent active TB was at least as safe and effective as the standard nine-month therapy for people living with HIV. The Phase 3 study, known as ACTG 5279, enrolled 3,000 individuals living with HIV around the world and randomly assigned a one-month or standard nine-month course of the anti-TB drug isoniazid. Both regimens were found to be safe and effective, though volunteers taking the one-month regimen were significantly more likely to adhere to the prescribed medication.
NIAID also supports research on opportunistic infections that can occur in cases of advanced stage HIV. When the immune system is damaged by uncontrolled HIV, fungi and other pathogens that the immune system would normally clear can lead to severe infections, like pneumocystis pneumonia or cryptococcal disease, that require immediate attention. To better address these infections when they occur, NIAID supports research to establish and refine best practices for diagnosing and treating these conditions.
Managing Non-Infectious Complications of HIV
The introduction of antiretroviral therapy (ART) and other treatment advances have transformed HIV care and the direction of treatment research over the past several decades. At the beginning of the HIV/AIDS pandemic in the 1980s, people often succumbed to opportunistic infections within years or even months of an HIV or AIDS diagnosis. Today, an individual diagnosed with HIV can expect to live to a near-normal lifespan with consistent use of effective HIV medications. As more people living with HIV progress through their fifties, sixties, and beyond, the medical community continues to learn more about how cardiovascular disease, type II diabetes, memory problems, cancer, and other conditions associated with aging may differ in people living with HIV.
Researchers have gained insight into how HIV influences individuals’ health over time through NIAID studies that follow cohorts of people living and aging with HIV, such as the START trial, the Women’s Interagency HIV Study, and the Multicenter AIDS Cohort Study. They have found that even when HIV is well-controlled with ART, immune cells undergo persistent activation that causes chronic inflammation in organs and systems throughout the body. Because inflammation is a key driver of many age-related illnesses, people with HIV are particularly prone to these conditions as they age. Also, while HIV medications ultimately preserve health and next-generation therapies have fewer side effects, long-term ART use has been linked to kidney abnormalities, osteoporosis, and other health problems.
Heart disease—the number one killer of men and women in the United States regardless of HIV status—is a particularly troubling complication of HIV infection. People living with HIV are 50 to 100 percent more likely to develop cardiovascular disease than people without HIV. This elevated risk is partially a result of chronic inflammation, which can harden blood vessels over time and increase one’s chances of experiencing heart attack and stroke. Additionally, some older HIV medications have side effects that can influence cholesterol levels, which can also contribute to cardiovascular disease.
To address the urgent need to prevent cardiovascular disease in people living with HIV, the National Heart, Lung, and Blood Institute (NHLBI) and NIAID launched the Randomized Trial to Prevent Vascular Events in HIV, or REPRIEVE, in 2015. The study has enrolled 7,500 participants between the ages of 40 and 75 in sites across the United States and abroad to determine whether a daily dose of a cholesterol-lowering statin can reduce the risk of cardiovascular disease in people living with HIV who would not normally be prescribed a statin. Researchers will also evaluate how this effect may differ for women living with HIV in an embedded study called Follow YOUR Heart.
Scientific Advances
NIH Clinical Trial of Tuberculous Meningitis Drug Regimen Begins
December 7, 2023A trial of a new drug regimen to treat tuberculous meningitis (TBM) has started enrolling adults and adolescents in several countries where tuberculosis (TB) is prevalent. The trial will include 330 participants aged 15 years and older who have or are likely to have TBM based on signs and symptoms, including people living with and without HIV. Because pregnant women are eligible to enroll in this…