Contact Information
Siu-Ping Turk, R.N., (301) 451-7661
lymedxstudies@niaid.nih.gov
Adriana R Marques, M.D, (301) 435-7244
amarques@niaid.nih.gov


Siu-Ping Turk, R.N., (301) 451-7661
lymedxstudies@niaid.nih.gov
Adriana R Marques, M.D, (301) 435-7244
amarques@niaid.nih.gov
Heidi Poppendeck, MPH
513-803-3078
heidi.poppendeck@cchmc.org
Kara Kliewer, PhD
513-636-4821
Kara.Kliewer@cchmc.org
Caeden Dempsey
301-761-7887
caeden.dempsey@nih.gov
Nana Kwatemaa, R.N.(301) 451-7820
nkwatemaa@niaid.nih.gov
Sergio D Rosenzweig, M.D.(301) 451-8971
srosenzweig@mail.nih.gov
Debra Reda, R.N. (301)496-9320
dreda@nih.gov
Joseph A Kovacs, M.D. (301) 496-9907
jkovacs@mail.nih.gov
Office of Patient Recruitment (OPR) 800-411-1222 ext TTY8664111010 prpl@cc.nih.gov
Established in 1942, the NIAID Laboratory of Infectious Diseases (LID) has a long history of discovering new agents of viral diseases and developing innovative vaccines and treatments such as FluMist, a nasal spray influenza vaccine; Synagis, a preventative treatment for respiratory syncytial virus; and Havrix, a hepatitis A vaccine licensed by GlaxoSmithKline.
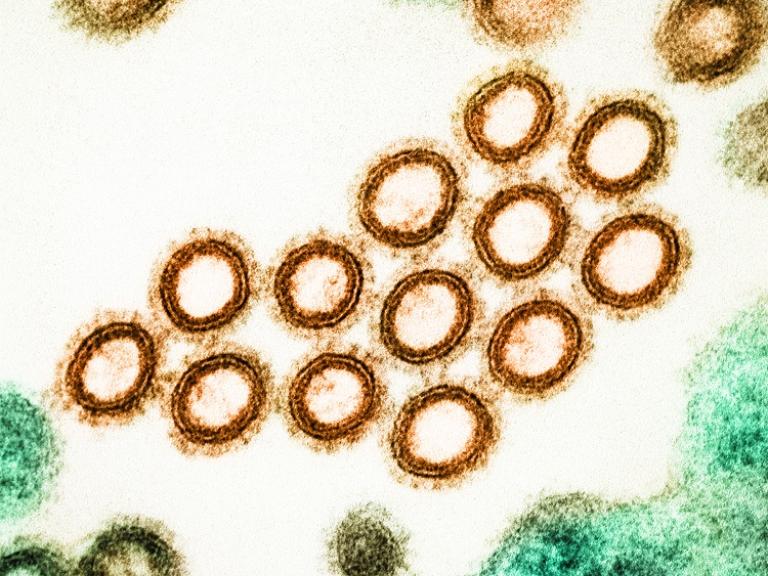
If you are healthy, have some time, and have an interest in helping researchers make discoveries about influenza (the flu), we need you for a screening study. The screening is used to determine eligibility for future studies that will help researchers learn more about the development and course of the flu virus.

This study seeks patients who are thought to have normal immune systems but who have been diagnosed with a viral infection that is unusually severe, prolonged, or persistent. This study will evaluate the participants' immune systems to determine why the infection developed.
The clinical trials and studies conducted by the Laboratory of Infectious Diseases Clinical Research Program change frequently, but you can see a list of all current clinical trials and studies being conducted by the Laboratory of Infectious Diseases Clinic on our Find a Clinical Trial page.
The LID Clinical Studies Unit (LID CSU) seeks to perform translational research studies to answer fundamental questions regarding human influenza and other emerging viral infections to inform and impact future vaccine and therapeutic design, while also making an effort to assist in evaluation of novel products that may impact human health.
In recent years, the LID CSU has initiated efforts to perform uncommon and difficult, but necessary clinical studies to evaluate novel vaccines and therapeutics particularly universal influenza vaccines in the human challenge model and extremely novel universal vaccines for vector borne disease like Dengue, Zika, and Leishmaniasis.

To me, science is better than magic. Through scientific research, we gain the ability to prevent or cure disease.
– Sara Jones
Read more about participating in the Participant's Guide to Clinical Trials
Read some Participant Testimonials to see more about what its like to participate.
You can also watch a series of short informational videos from Health and Human Services about participating in clinical trials.
For questions about participating in clinical studies, contact:
Office of Patient Recruitment
Toll Free: 1-800-411-1222
TTY: 1-866-411-1010
Se habla español. Email: prpl@mail.cc.nih.gov
The NIAID Primary Immune Deficiency (PID) Clinic is located at the National Institutes of Health (NIH) Clinical Center and is dedicated to research on the more than 200 forms of primary immune deficiency diseases affecting approximately 500,000 people in the United States.
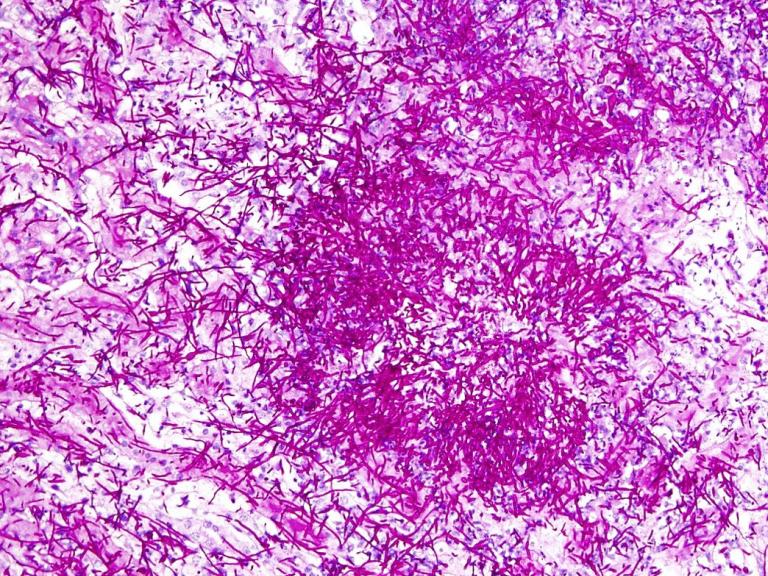
The study is investigating why some people are susceptible to IFIs even without underlying immune deficiency. It will explore genetic and immune system factors that may contribute to the condition.
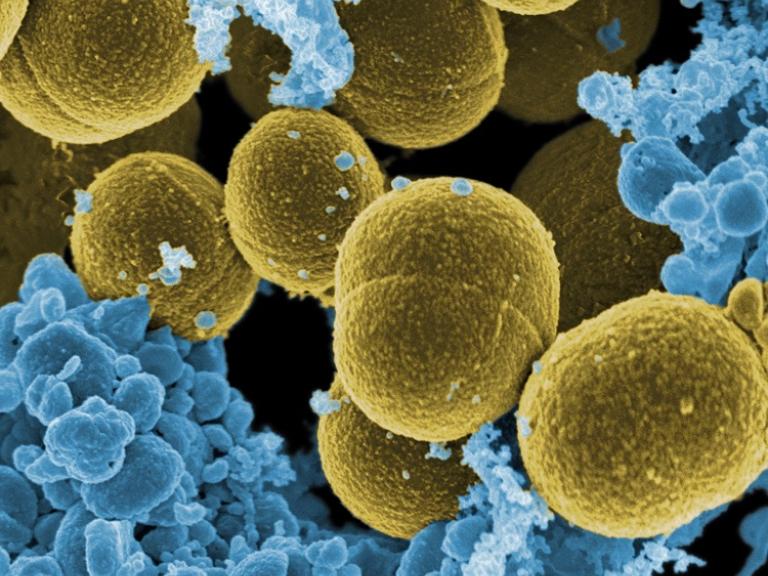
Chronic granulomatous disease (CGD) is a genetic disorder in which white blood cells called phagocytes are unable to kill certain types of bacteria and fungi. NIAID aims to improve diagnosis, explore new treatments and preventions, and facilitate genetic counseling for this primary immune deficiency disease.
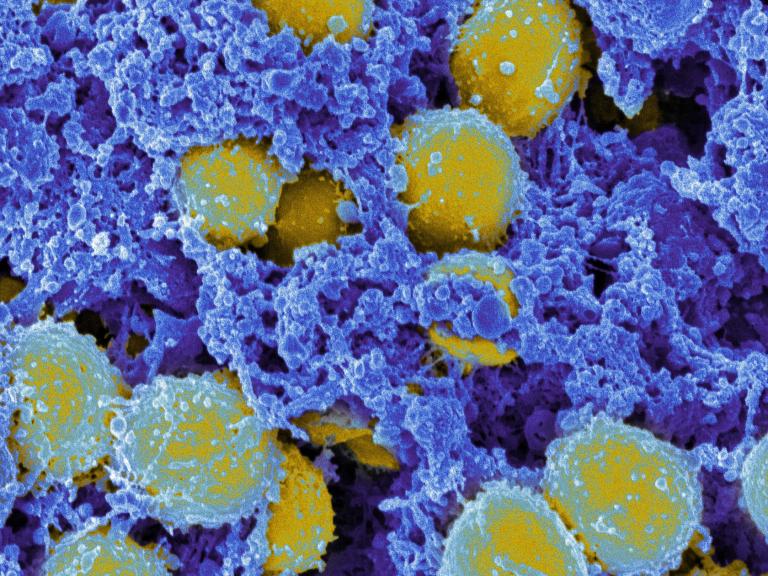
STAT3 dominant-negative disease — also known as Job’s Syndrome—has widespread deleterious effects on patients’ health. NIAID-supported research seeks to determine the effect of STAT3DN on the immune system, including which immune cells and responses are affected and how these abnormalities translate into patients’ symptoms.
The clinical trials and studies conducted by the Primary Immune Deficiency Clinic change frequently, but you can see a list of all current clinical trials and studies being conducted by the Primary Immune Deficiency Clinic on our Find a Clinical Trial page.
The clinic accepts patients who are at least 2 years old who have a known or suspected primary immune deficiency disease.
If you are interested in coming to the PID Clinic, discuss this option with your personal physician or specialist.
You must be referred to the PID Clinic by your doctor. Your doctor will need to send the PID Clinic staff the following information:
Send the requested information and records by FedEx to:
National Institutes of Health
10 Center Drive
Building 10, Room 12C-103
Bethesda, MD 20892-1899
Attn: Carla Williams, RN
Phone: 301–496–4000
Our clinicians will notify your doctor and you of their decision by mail in about 6 to 8 weeks. If our clinicians determine that you would benefit from coming to the PID Clinic, you and your family will then be invited. Our clinical staff will send you a copy of the NIH Patient Handbook and a copy of the NIH Screening Consent prior to your visit to NIH.
Your visit to the clinic is free, so there is no need to worry if your insurance will cover your visit. However, your initial travel expenses will not be covered.
For those arriving from outside the Washington, DC, metro area, some overnight housing is available on the NIH campus at the Children’s Inn. There are also many hotels and motels convenient to public transportation in the area.
Plan to stay for at least one full day (approximately eight hours) of testing and evaluation. Some diagnostic tests take two days to complete, so you may need to return to the clinic for a half-day follow-up visit.
During your visit, you can expect the following:
Any patient of the PID clinic is eligible to receive genome sequencing and associated services through the NIAID Centralized Sequencing Program. Patients interested in participating in the sequencing process can visit the NIAID Centralized Sequencing Program page for eligibility criteria and contact information.
Of Special Interest to Parents (NIH Clinical Center)
For Parents and Children (NIH)
The NIH Clinical Center provides many services to its visitors, including counseling, a business center, and recreation. For details, visit the NIH Clinical Center Patient Services website.
Our staff is committed to helping you better manage your disease.
After your exam at the clinic is complete, you may be offered the opportunity to participate in ongoing clinical trials at NIAID. Only our clinicians can determine if you are eligible to participate.
Alternatively, you may be informed of primary immune deficiency diseases studies for which you may be eligible at clinical centers outside of NIAID.
Learn more about NIAID's research on primary immune deficiency diseases.

The best part of participating in the trial was seeing the staff during each visit: they were so upbeat, kind, and thankful. I felt as though I was part of a team that would change the world—and I know it will.
– Tony Lee
Read more about participating in the Participant's Guide to Clinical Trials
Read some Participant Testimonials to see more about what its like to participate.
You can also watch a series of short informational videos from Health and Human Services about participating in clinical trials.
National Institutes of Health
10 Center Drive
Building 10, Room 12C-103
Bethesda, MD 20892-1899
Attn: Carla Williams, RN
Phone: 301–496–4000
The Vaccine Research Center evaluates candidate vaccines and monoclonal antibodies targeting HIV, influenza, Ebola, and other emerging infections.

This is a study of an experimental product called CAP256J3LS. This study will provide more information about prevention or treatment of human immunodeficiency virus (HIV) infection.

Volunteering for this study will help us provide more information about flu vaccines that may one day lead to a universal flu vaccine that would be effective against all influenza strains.
The clinical trials and studies conducted by the Vaccine Research Center change frequently, but you can see a list of all current clinical trials and studies being conducted by VRC on our Find a Clinical Trial page.
The Vaccine Research Center is looking for adults 18 years of age and older to participate in trials evaluating vaccine and monoclonal antibodies
Once you agree to participate, we will follow the steps below.
If you are interested in volunteering to participate in a clinical study, please call us at 866-833-LIFE (5433) or email us at vaccines@nih.gov. We will send you information about our clinical studies and follow up with you to answer any questions.
Or fill out the volunteer information form and we will match you with studies and contact you.
Learn more about the work being done at NIAID’s Vaccine Research Center.

Based on my personal experiences, I encourage people to look into the clinical trials at the VRC. You really cannot ask for nicer or more dedicated and knowledgeable staff than those at the VRC, and they appreciate all of their volunteers. Give it a shot and be a part of something truly important!
– Peter Hubbard
Read more about participating in the Participant's Guide to Clinical Trials
Read some Participant Testimonials to see more about what its like to participate.
You can also watch a series of short informational videos from Health and Human Services about participating in clinical trials.
For more information or to volunteer for a vaccine research study
NIAID and the National Institutes of Health (NIH) Warren G. Magnuson Clinical Center conduct clinical research on HIV, chronic viral hepatitis (hepatitis B and C), emerging infectious diseases, and other immunological disorders.
This joint research program is part of the NIH, the world's largest medical research institution, which is operated by the federal government.
Long-Term Non-Progressors - a small group of people who are HIV positive yet remain healthy for long periods of time without medications - can help researchers at NIH determine how their immune systems control HIV disease. This knowledge is critical for developing new treatments and vaccines.
The clinical trials and studies conducted by the HIV and Emerging Infectious Diseases Program change frequently, but you can see a list of all current clinical trials and studies being conducted by the HIV and Emerging Infectious Diseases Clinic on our Find a Clinical Trial page.
Some of the research studies at the NIH Clinical Center involve promising new treatments that may directly benefit patients.
A clinical trial (also clinical research) is a research study in which people participate as volunteers.
A research study is a scientific investigation to achieve a complete understanding of a topic.
Carefully conducted clinical trials are the fastest and safest way to find
There are strict rules for clinical trials, which are monitored by
Participants in clinical trials are
Participants in clinical trials can
Learn more about the research conducted at NIAID to fight HIV and other emerging infectious diseases

I’ve realized that our only hope of truly curbing [the HIV/AIDS] pandemic is the discovery of a vaccine. This is why I fight.
– Kymone Freeman
Read more about participating in the Participant's Guide to Clinical Trials
Read some Participant Testimonials to see more about what its like to participate.
You can also watch a series of short informational videos from Health and Human Services about participating in clinical trials.
Volunteer or get more information
Toll free: 1-800-411-1222
TTY: 1-866-411-1010
Se habla español
Email: prpl@mail.cc.nih.gov
Researchers want to study the immune systems of people with DCM or RCM, to learn more about the disease and the best ways to treat it. They also want to learn more about the types of people that get DCM or RCM and about the fungus that causes it.
Merertu Tesso (301)-402-7831
tessom@mail.nih.gov
Steven M Holland, M.D. (301) -402-7684
sholland@mail.nih.gov