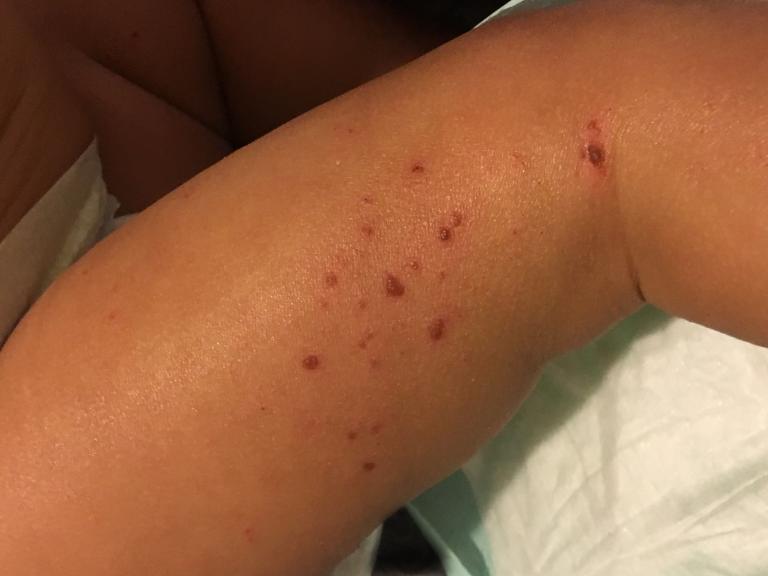
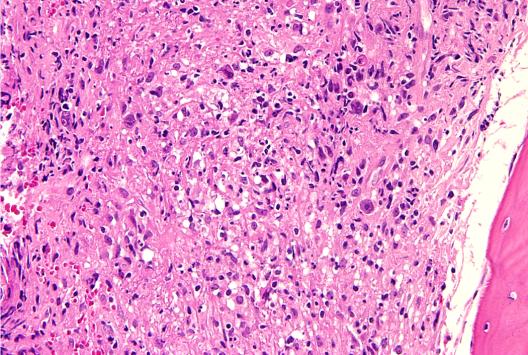
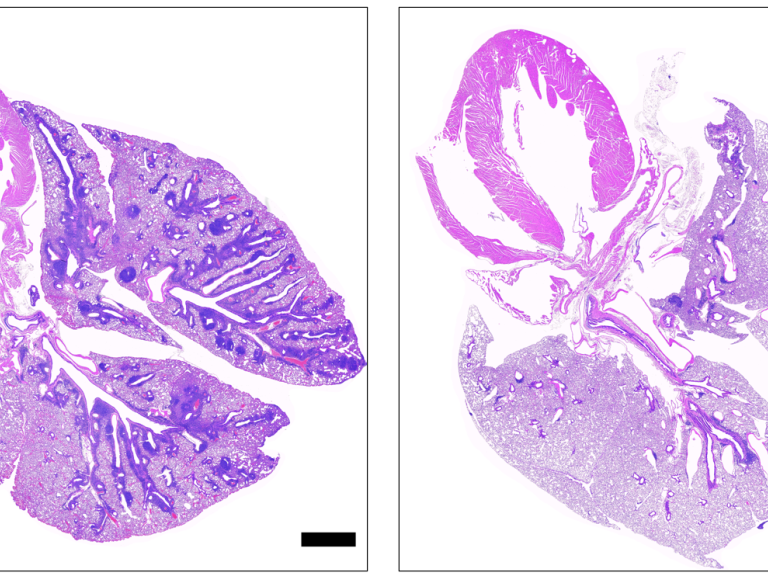
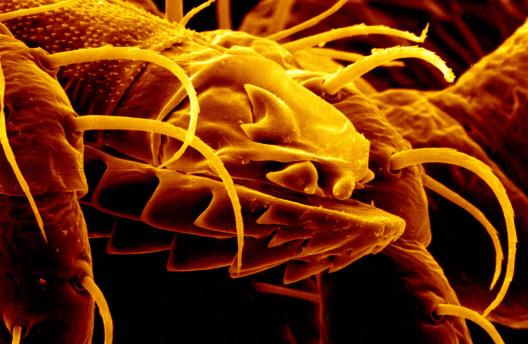
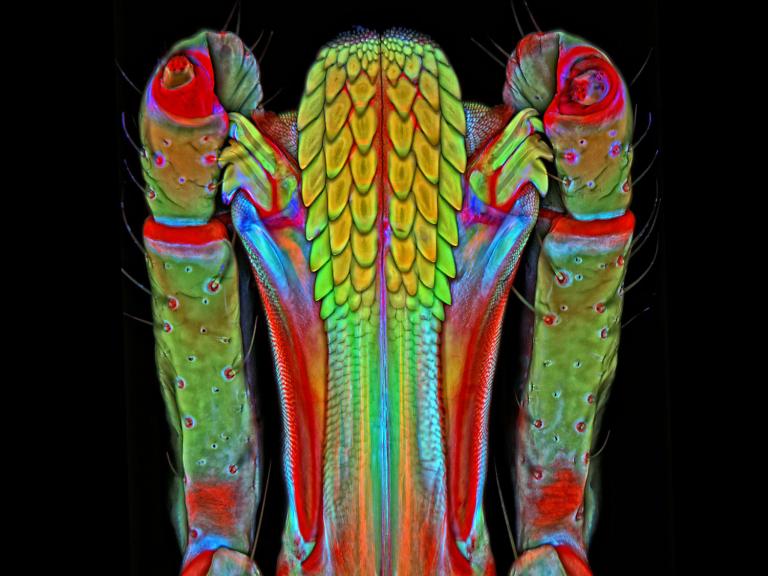
Leishmaniasis is a parasitic disease transmitted by the bites of infected sand flies. It is found in nearly 88 countries, from rain forests in Central and South America to deserts in the Middle East and west Asia. Some cases of the disease have also appeared in Mexico and Texas. The disease takes several different forms, including the most common cutaneous leishmaniasis, which causes skin lesions, and the more severe visceral leishmaniasis (also known as kala azar), which affects internal organs such as the spleen, liver, and bone marrow.
Why Is the Study of Leishmaniasis a Priority for NIAID?
The World Health Organization estimates there are between 600,000 and 1 million new cases of cutaneous leishmaniasis and 50,000-70,000 new cases of visceral leishmaniasis in the world each year. Leishmaniasis not only affects people who live in countries where the disease is endemic but also poses a risk to people who travel in those areas. According to the World Health Organization (WHO), 90 percent of all cutaneous leishmaniasis cases occur in Afghanistan, Brazil, Iran, Peru, Saudi Arabia, and Syria.
How Is NIAID Addressing This Critical Topic?
NIAID conducts and supports leishmaniasis research to advance the understanding of all aspects of the disease, including the different species of disease-causing Leishmania parasites, the varieties and biology of sand flies that transmit the parasites to animals and humans, and how the host immune system responds to the infection. NIAID also supports development of therapeutics for treatment.
Related Public Health and Government Information
To learn about risk factors for leishmaniasis and current prevention and treatment strategies visit the MedlinePlus leishmaniasis site.
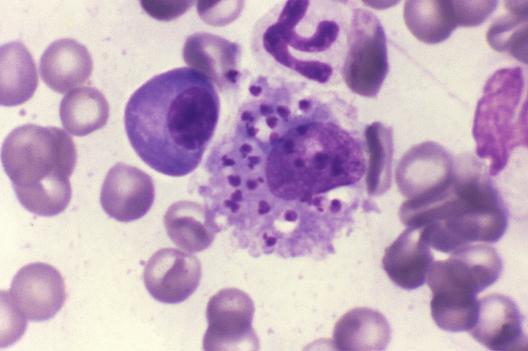
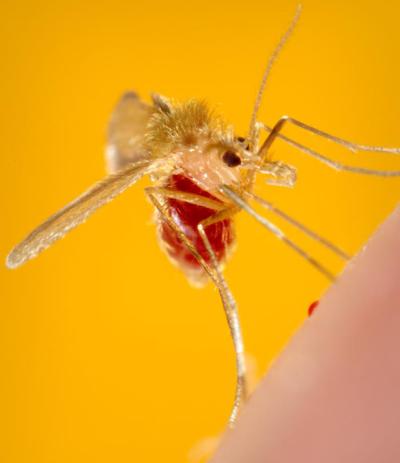
Phlebotomus papatasi sand fly.