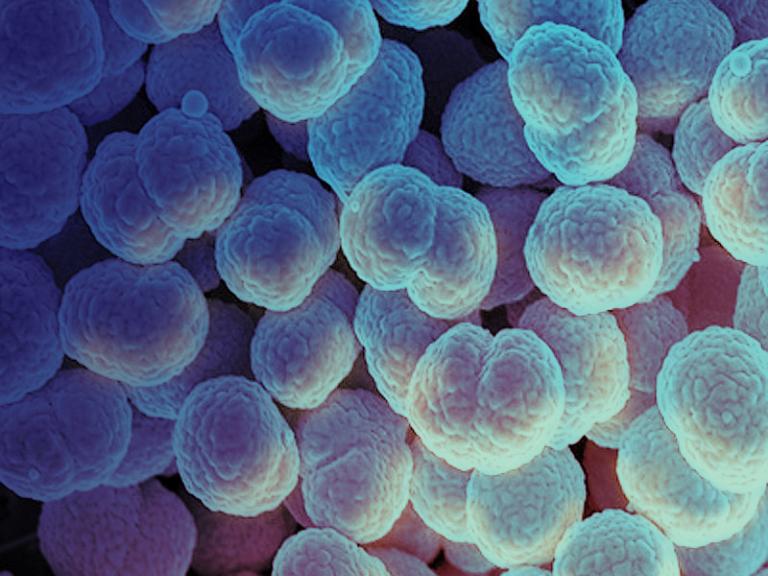
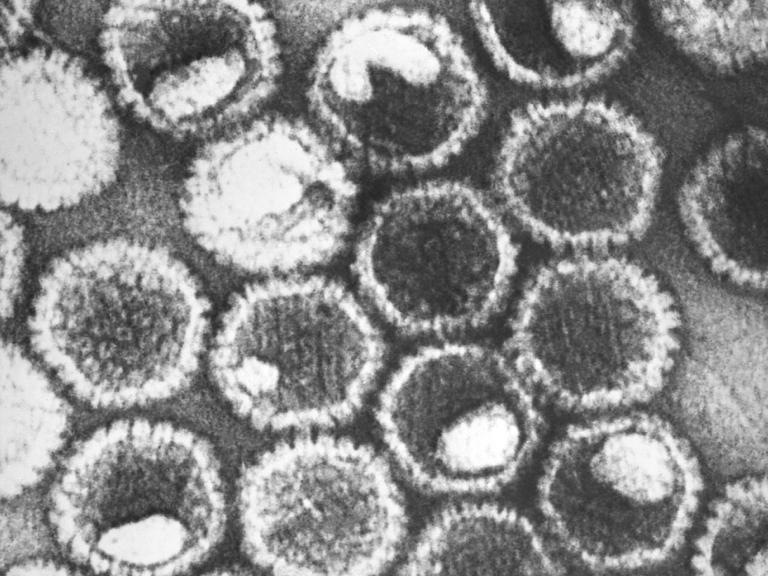
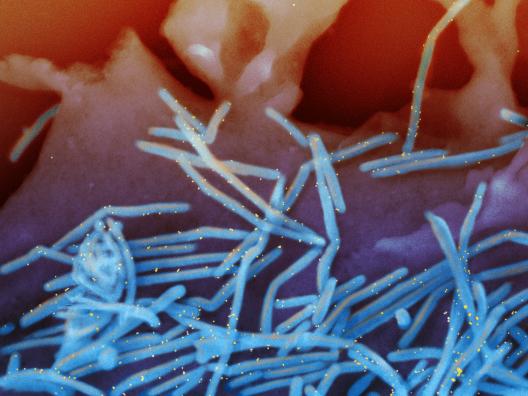
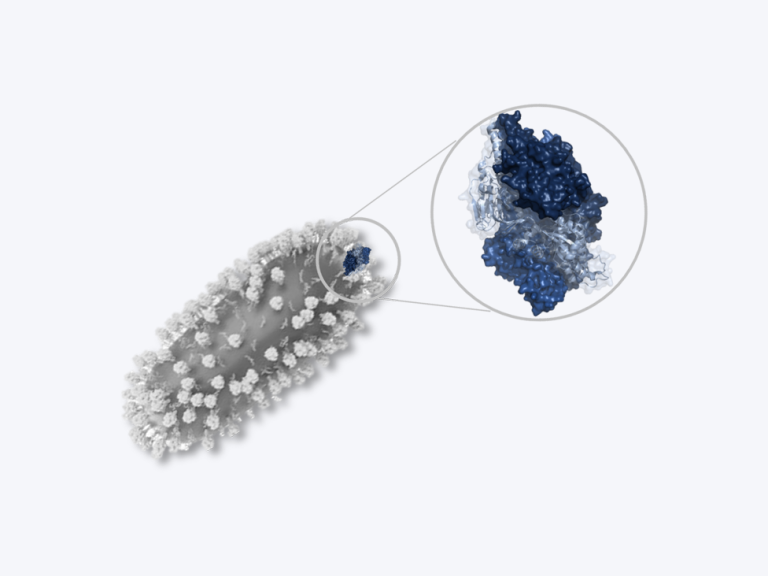
Coronaviruses are a large family of viruses that usually cause mild to moderate upper-respiratory tract illnesses in humans. However, three coronaviruses have caused more serious and fatal disease in people: SARS coronavirus (SARS-CoV), which emerged in November 2002 and causes severe acute respiratory syndrome (SARS); MERS coronavirus (MERS-CoV), which emerged in 2012 and causes Middle East respiratory syndrome (MERS); and SARS-CoV-2, which emerged in 2019 and causes coronavirus disease 2019 (COVID-19).
Building on previous research on SARS and MERS, NIAID scientists and NIAID-supported researchers mobilized quickly to develop COVID-19 therapeutics, vaccines and diagnostics. Researchers continue to conduct basic research to understand how coronaviruses infect cells and causes disease, and what interventions can detect, prevent and stop the spread of disease.
Public Health and Government Response to COVID-19
- National Institutes of Health (NIH) COVID-19 Research
- Coronavirus (COVID-19) health information from the Centers for Disease Control & Prevention (CDC).
- Health and Human Services Administration for Strategic Preparedness & Response
- NIH Community Engagement Alliance (CEAL)
- To learn about risk factors for coronaviruses and current prevention and treatment strategies visit the MedlinePlus COVID-19 (Coronavirus Disease 2019) site.
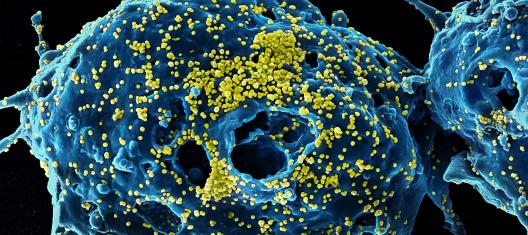
Highlights
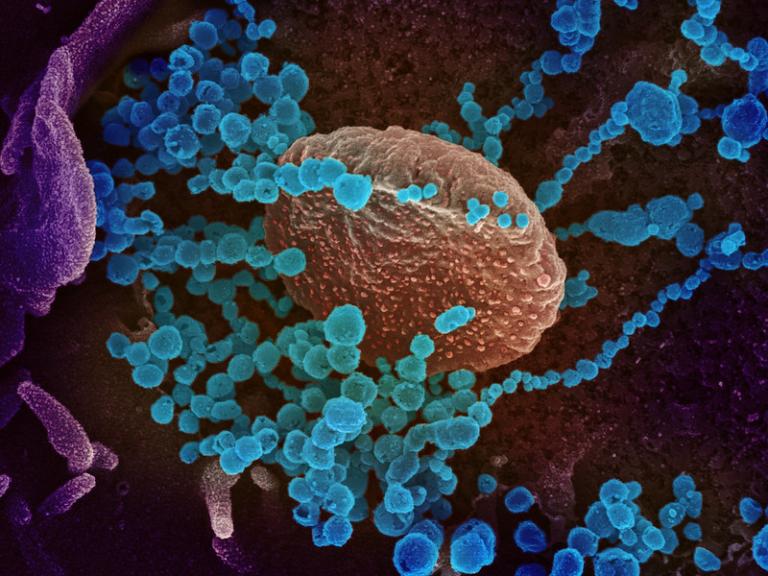
First Webinar of Long COVID Treatment Initiative Highlights Early Progress
NIAID and the Foundation for the National Institutes of Health (FNIH) launched its first in a series of online webinars highlighting recent progress in the new Researching COVID to Enhance Recovery - Treating Long COVID (RECOVER-TLC) program.
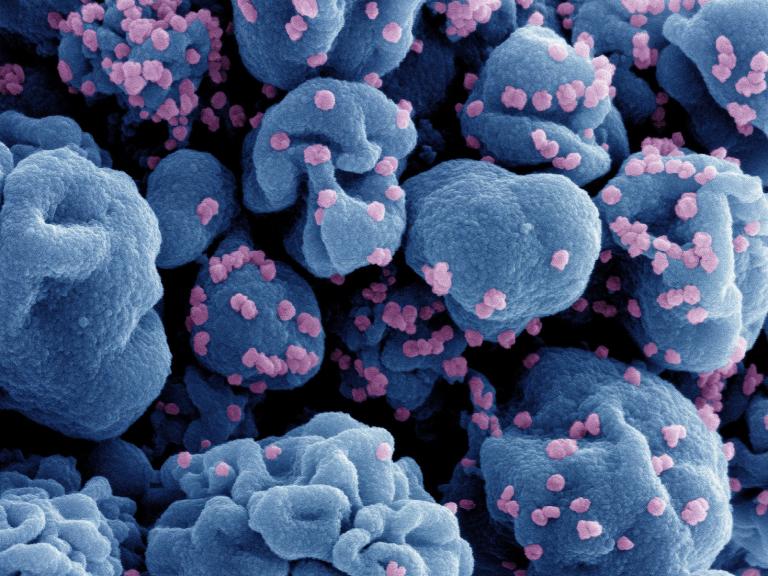
NIH-Sponsored Trial of Nasal COVID-19 Vaccine Opens
A Phase 1 trial testing the safety of an experimental nasal vaccine that may provide enhanced breadth of protection against emerging variants of SARS-CoV-2 is now enrolling healthy adults at three sites in the United States.
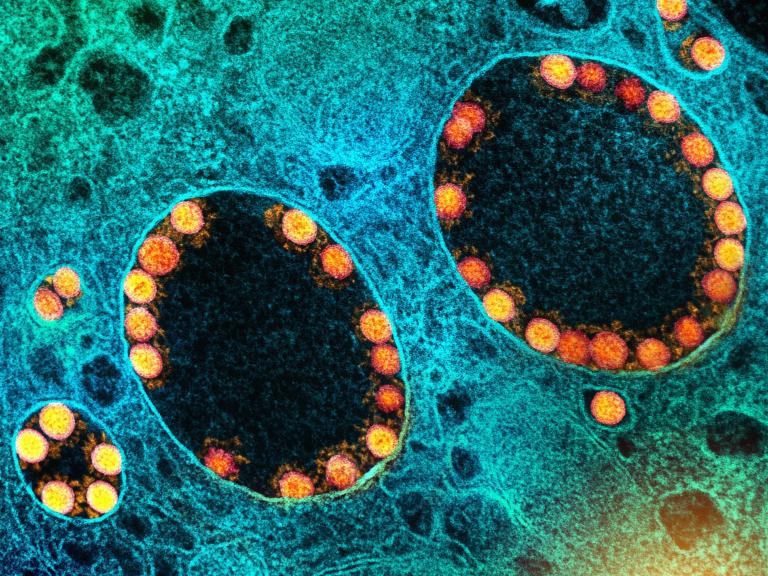
COVID-19 Vaccination and Boosting During Pregnancy Protects Infants for Six Months
Women who receive an mRNA-based COVID-19 vaccination or booster during pregnancy can provide their infants with strong protection against symptomatic COVID-19 infection for at least six months after birth.