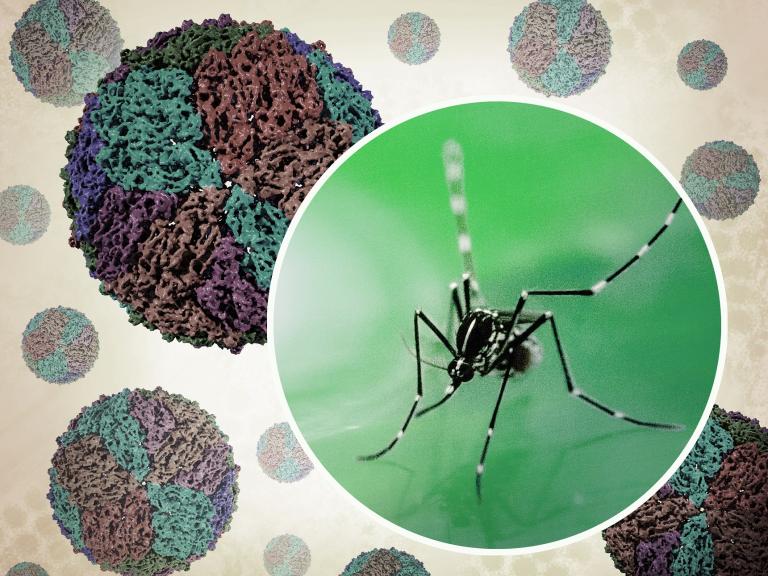
Zika virus, like other members of the flavivirus family including dengue and West Nile virus, is most typically transmitted to humans through the bite of infected Aedes aegypti mosquitoes. Less commonly, Zika virus can be spread from person to person through sexual intercourse. Most people who become infected with Zika virus do not become sick; but about 1 in 5 people may develop such symptoms as fever, rash, and conjunctivitis (reddened eyes). People who become infected while pregnant may transmit the virus to the fetus, which can result in very serious birth defects, including microcephaly (unusually small head).
Zika virus was discovered in the Zika forest in Uganda in 1947. In 2015, cases were reported in Brazil and an outbreak of Zika virus disease followed in South and Central America as well as the Caribbean. The first cases of locally transmitted Zika virus in the continental United States were confirmed in Florida in July 2016. No cases of mosquito-transmitted Zika virus have been detected in the United States since 2018.
NIAID supports research to better understand Zika virus, the disease it causes, and ways to combat it, including research on diagnostics to rapidly determine if someone is or has been infected with Zika and to distinguish from other flaviviruses.
Related Public Health and Government Information
NIAID research helps us learn more about the Zika virus to help those affected. Read information from the Centers for Disease Control and Prevention and the National Library of Medicine's Zika Virus Health Information Resource Guide for more information on where the current risks are and other research initiatives world wide.
Highlights

NIAID and Cuban Scientists Gather to Discuss Global Health Challenges
Recent disease outbreaks in the Americas led U.S. and Cuban scientists to hold a meeting Feb. 14-16 on Addressing Global Health Challenges Through Scientific Innovation and Biomedical Research.

Promising Advances for Antibody Treatment of Viruses that Cause Neurologic and Arthritic Diseases
NIAID scientists and colleagues are one step closer to developing a safe and effective therapy against alphaviruses with the identification of SKT05, a monoclonal antibody (mAb) derived from macaques vaccinated with virus-like particles (VLPs) representing three encephalitic alphaviruses.