Table of Contents
See the main index of Decision Trees or Research Using Human Subjects
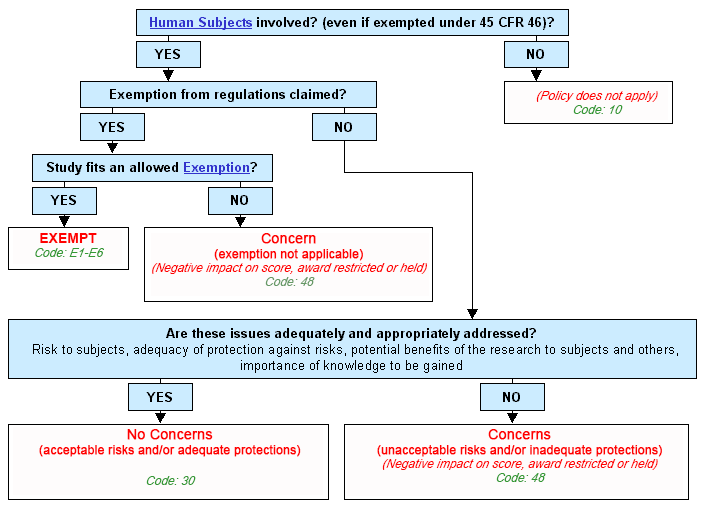
Graphical Flowchart
Credit:
NIAID
Text Version of Flowchart
Step 1. Are human subjects involved? (even if exempted under 45 CFR Part 46)?
- If yes, continue to Step 2.
- If no:
- The policy does not apply.
- Human Subjects Code: 10.
- End.
Step 2. Does the study claim an exemption to regulations?
Step 3. Does the study fit an allowed exemption?
- If yes:
- Exempt.
- Human Subjects Code: X1-X8.
- End.
- If no:
- Concern. Exemption not applicable.
- Negative impact on score, award restricted or held.
- Human Subjects Code: 48.
- End.
Step 4. Are these issues adequately and appropriately addressed? Risk to subjects, adequacy of protection against risks, potential benefits of the research to subjects and others, importance of knowledge to be gained
- If yes:
- No concerns. Acceptable risks and/or adequate protections.
- Human Subjects Code: 30.
- End.
- If no:
- Concerns. Unacceptable risks and/or inadequate protections.
- Negative impact on score, award restricted or held.
- Human Subjects Code: 48.
- End.
Summary of Codes
- Human subjects protection codes
- 10—Human subjects not involved (not clinical research)
- 30—Human subjects involved, No Concerns (or only minor problems)
- 48—At time of award, restrictions will apply
- Exemption codes: Research involves...
- X1—Normal educational practices in an educational setting
- X2—Educational tests unlinkable to individuals, with no risks from disclosure
- X3—Benign behavioral interventions
- X4—Existing data/specimens, publicly available or unlinkable to individuals
- X5—Demonstration projects concerning public benefit or service programs
- X6—Taste and quality evaluation of foods without additives exceeding regulated levels
- X7—Storage of identifiable information or biospecimens for secondary research use.
- X8—Secondary research use of identifiable information or biospecimens.

