Related SOP: Investigator-Initiated Clinical Trial Planning and Implementation Awards SOP
Credit:
NIAID
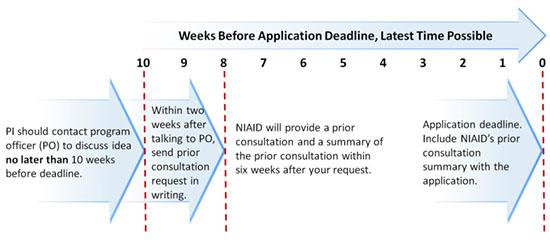
We strongly advise you to perform these steps well in advance of the dates listed.
- Principal investigator (PI) should contact program officer (PO) to discuss project idea no later than 10 weeks before deadline. For the extended clinical trial R01 opportunity or U01 applications with budget requests of $1,000,000 or more in direct costs in any year, allow 12 weeks instead.
- NIAID will provide prior consultation and a summary within six weeks after your request.
- Include the NIAID prior consultation summary letter with the application.
For general information on investigator-initiated clinical trial planning and implementation awards, go to Investigator-Initiated Clinical Trial Resources.

