Pathogen-Host Cell Biology Section
Frank R. DeLeo, Ph.D.
Chief, Laboratory of Bacteriology
Chief, Pathogen-Host Cell Biology Section

Major Areas of Research
- Neutrophil biology and function
- Evasion of innate immunity by bacterial pathogens
- Host interactions with antibiotic-resistant bacteria
Program Description
Although most bacteria are killed readily by neutrophils, pathogens such as Staphylococcus aureus have evolved mechanisms to circumvent destruction by these key innate immune cells and thereby cause human infections. A better understanding of the bacteria-neutrophil interface at the cell and molecular levels will provide information critical to our understanding, treatment, and control of disease caused by bacterial pathogens.
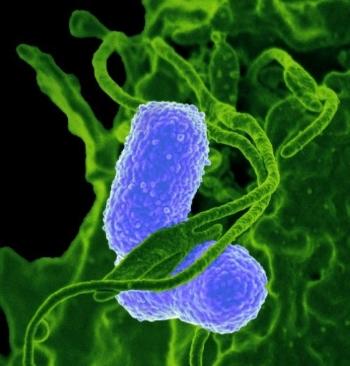
Interaction of Klebsiella pneumoniae with a human neutrophil.
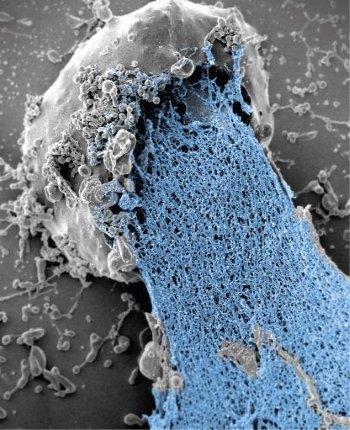
Formation of a neutrophil extracellular trap.
The long-term objective of our research is to promote development of enhanced diagnostics, better prophylactic agents, and new treatments for emerging bacterial pathogens such as community-associated methicillin-resistant S. aureus (CA-MRSA) and carbapenem-resistant Klebsiella pneumoniae. To achieve that objective, the Pathogen-Host Cell Biology Section does the following:
- Conducts a systematic molecular dissection of steps involved in the pathogen-host interaction, with emphasis on the interaction of bacterial pathogens with human neutrophils
- Investigates mechanisms mediating evasion of innate immunity by bacterial pathogens
- Identifies new virulence genes involved in the pathogenesis of infections caused by pathogens of special interest
- Uses animal infection models and (if possible) human specimens to test hypotheses developed from in vitro analyses
Biography
Education
Ph.D., 1996, Montana State University
Dr. DeLeo received his Ph.D. in microbiology from Montana State University in 1996, studying the molecular basis of superoxide generation by human neutrophils. He did his postdoctoral training in the area of innate immunity and infectious diseases in the Department of Medicine at the University of Iowa (1996–2000). Dr. DeLeo joined the staff at the NIAID Rocky Mountain Laboratories in 2000 as a tenure-track investigator. He served previously as Acting Chief (2007-2013) and Chief (2013-2015) of the Laboratory of Human Bacterial Pathogenesis. Dr. DeLeo was appointed to the NIH Senior Biomedical Research Service (2011-2017) and elected as an American Academy of Microbiology Fellow in 2017. He is currently Chief of the Laboratory of Bacteriology.
Editorial Boards
- Infection and Immunity
- Journal of Innate Immunity
Selected Publications
Malachowa N, McGuinness W, Kobayashi SD, Porter AR, Shaia C, Lovaglio J, Smith B, Rungelrath V, Saturday G, Scott DP, Falugi F, Missiakas D, Schneewind O, DeLeo FR. Toward Optimization of a Rabbit Model of Staphylococcus aureus (USA300) Skin and Soft Tissue Infection. Microbiol Spectr. 2022 Apr 27;10(2):e0271621.
Opoku-Temeng C, Malachowa N, Kobayashi SD, DeLeo FR. Innate Host Defense against Klebsiella pneumoniae and the Outlook for Development of Immunotherapies.
J Innate Immun. 2022;14(3):167-181.
Rungelrath V, Porter AR, Malachowa N, Freedman BA, Leung JM, Voyich JM, Otto M, Kobayashi SD, DeLeo FR. Further Insight into the Mechanism of Human PMN Lysis following Phagocytosis of Staphylococcus aureus. Microbiol Spectr. 2021 Oct 31;9(2):e0088821.
Hesse S, Malachowa N, Porter AR, Freedman B, Kobayashi SD, Gardner DJ, Scott DP, Adhya S, DeLeo FR. Bacteriophage Treatment Rescues Mice Infected with Multidrug-Resistant Klebsiella pneumoniae ST258. mBio. 2021 Feb 23;12(1):e00034-21.
Rungelrath V, DeLeo FR. Staphylococcus aureus, Antibiotic Resistance, and the Interaction with Human Neutrophils. Antioxid Redox Signal. 2021 Feb 20;34(6):452-470.
Research Group
Bacterial pathogenesis and host defense

