Pathogen Molecular Genetics Section
Michael Otto, Ph.D.
Chief, Pathogen Molecular Genetics Section
Specialty(s): Infectious Disease

Major Areas of Research
- Staphylococcal infection and colonization
- Bacterial interactions with the host and the host microbiota
- Antibiotic resistance in Staphylococcus aureus (MRSA)
- Biofilm development and infection
Program Description
Our research focuses on Gram-positive bacteria (staphylococci, enterococci, Cutibacterium acnes, etc.) and their interaction with humans and with other bacteria within the human microbiota. Most work is performed on staphylococci, bacteria that colonize epithelial surfaces of humans and other mammals. Most have a benign, or as more recently discovered, even beneficial, relationship with the host. However, some of them can cause serious infections after breaching through the epithelia of the skin or other organs.
The most dangerous pathogen of the genus is Staphylococcus aureus, which is a leading cause of hospital- and community-associated infections, such as infections of the lung, skin, bones, or blood. Many of them can be fatal. We are interested in mechanisms of staphylococcal pathogenesis with a current focus on peptide toxins (phenol-soluble modulins, PSMs) and quorum-sensing.
Staphylococci frequently cause device-associated infections, which characteristically involve biofilms and can develop into dangerous blood infections in patients undergoing surgery or those with underlying conditions. We have performed intensive research on staphylococcal biofilm formation, including on contributing factors such as exopolysaccharides, on biofilm regulation, and on dispersal factors. Our focus is on in-vivo relevance of biofilm formation mechanisms.
Antibiotic resistance (such as in methicillin-resistant S. aureus, MRSA) is a major problem associated with staphylococcal infections. Our laboratory investigates mechanisms of virulence in MRSA and other antibiotic-resistant staphylococci with the goal of developing anti-virulence treatment options.
We are also performing research on the interaction of Staphylococcus bacteria with the host and with other microorganisms within the microbiota of the human intestine and skin. Part of this research includes the evaluation of probiotic interactions and their translational use.
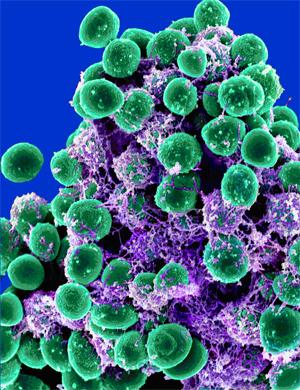
Scanning electron microscopy of Staphylococcus epidermidis cluster embedded in exopolysaccharide matrix.
Biography
Education
Ph.D., 1998, M.S., 1993, University of Tübingen, Germany
Dr. Otto received his M.S. in biochemistry in 1993 from the University of Tübingen, Germany. In 1998, he earned his Ph.D. in microbiology from the same institution. Dr. Otto joined the Laboratory of Human Bacterial Pathogenesis in July 2001 as a principal investigator. In 2008, he became a tenured senior investigator and moved his laboratory to the NIH Bethesda main campus.
Dr. Otto serves on several editorial advisory boards and is a section editor (Gram-positive bacteria) at PLoS Pathogens.
Selected Publications
Zheng Y, Hunt RL, Villaruz AE, Fisher EL, Liu R, Liu Q, Cheung GYC, Li M, Otto M. Commensal Staphylococcus epidermidis contributes to skin barrier homeostasis by generating protective ceramides. Cell Host Microbe. 2022 Mar 9;30(3):301-313.e9.
Nguyen TH, Cheung GYC, Rigby KM, Kamenyeva O, Kabat J, Sturdevant DE, Villaruz AE, Liu R, Piewngam P, Porter AR, Firdous S, Chiou J, Park MD, Hunt RL, Almufarriji FMF, Tan VY, Asiamah TK, McCausland JW, Fisher EL, Yeh AJ, Bae JS, Kobayashi SD, Wang JM, Barber DL, DeLeo FR, Otto M. Rapid pathogen-specific recruitment of immune effector cells in the skin by secreted toxins. Nat Microbiol. 2022 Jan;7(1):62-72.
Piewngam P, Chiou J, Ling J, Liu R, Pupa P, Zheng Y, Otto M. Enterococcal bacteremia in mice is prevented by oral administration of probiotic Bacillus spores. Sci Transl Med. 2021 Nov 24;13(621):eabf4692.
He L, Le KY, Khan BA, Nguyen TH, Hunt RL, Bae JS, Kabat J, Zheng Y, Cheung GYC, Li M, Otto M. Resistance to leukocytes ties benefits of quorum sensing dysfunctionality to biofilm infection. Nat Microbiol. 2019 Jul;4(7):1114-1119.
Piewngam P, Zheng Y, Nguyen TH, Dickey SW, Joo HS, Villaruz AE, Glose KA, Fisher EL, Hunt RL, Li B, Chiou J, Pharkjaksu S, Khongthong S, Cheung GYC, Kiratisin P, Otto M. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018 Oct;562(7728):532-537.
Chatterjee SS, Joo HS, Duong AC, Dieringer TD, Tan VY, Song Y, Fischer ER, Cheung GY, Li M, Otto M. Essential Staphylococcus aureus toxin export system. Nat Med. 2013 Mar;19(3):364-7.
Research Group
The Pathogen Molecular Genetics section investigates physiology and host interaction of Gram-positive pathogens with a focus on Staphylococcus aureus and coagulase-negative staphylococci. We are also investigating the interactions of commensal staphylococci with other bacteria within the human microbiome and the potential of probiotic approaches to control bacterial infections.


