Biology of Vector-Borne Viruses Section
Marshall E. Bloom, M.D.
Associate Director for Science Management, Rocky Mountain Laboratories
Chief, Biology of Vector-Borne Viruses Section

Major Areas of Research
- Structural biology of tick-borne flaviviruses in vertebrate and arthropod systems
- Biology and molecular pathogenesis of acute and persistent tickborne flavivirus infections
- Viral and host determinants of effective vertical (through the tick life stages) and horizontal (from tick to mammalian host) transmission
Program Description
The laboratory investigates the pathogenesis of viruses belonging to the tickborne encephalitis (TBE) virus complex of flaviviruses. Endemic throughout much of the northern hemisphere, these viruses can cause severe encephalitis, meningitis, or hemorrhagic fevers with relatively high mortality rates. Our research utilizes in vitro and animal models (mouse and tick) to examine the biology of acute and persistent infection in mammalian and arthropod systems. Understanding these aspects of virus biology will increase understanding of these important pathogens and may provide targets for the design of countermeasures. Methods employed include confocal microscopy, electron microscopy, electron cryotomography, immunohistochemistry, microarray analysis, nucleic acid sequencing, and molecular virology.
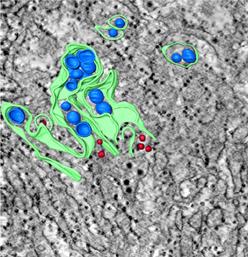
Figure 1. Reconstructed electron tomogram depicting endoplasmic reticulum (green), viral induced vesicles (blue), and virions (red) in Vero cells infected for 40 hours with a tickborne flavivirus. The vesicles are thought to form by budding into cisternae of the endoplasmic reticulum and to contain viral replication complexes, sequestering them from intracellular antiviral sensors.
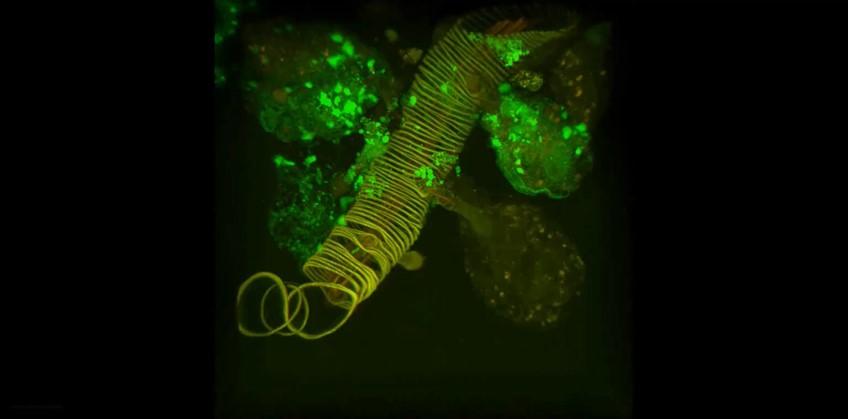
An image of an ex vivo culture of an Ixodes scapularis salivary gland infected with a GFP-expressing tickborne flavivirus. The image shows a salivary duct and several acini. The fluorescent signal indicates virus infection of acinar cells.
Biography
Education
M.D., 1971, Washington University School of Medicine, St. Louis, MO
Dr. Bloom received his M.D. in 1971 from Washington University School of Medicine in St. Louis, Mo., and then joined the Rocky Mountain Laboratories (RML) of NIAID in 1972 as a research associate. From 1975 to 1977, he was a postdoctoral fellow in the NIAID Laboratory of the Biology of Viruses on the NIH campus in Bethesda, MD. He returned to RML as a tenured investigator in 1977 and was a charter member of the Laboratory of Persistent Viral Diseases (now Laboratory of Neurological Infections and Immunity). He is a world expert in the molecular biology and pathogenesis of parvoviruses and is considered an authority in biocontainment. In 2004, Dr. Bloom’s research group changed its focus to the pathogenesis of tickborne flaviviruses. In 2002, Dr. Bloom was appointed associate director for RML in NIAID’s Division of Intramural Research, and among his duties have been program supervision of the permitting, construction, and staffing of NIAID's first biosafety level-4 facility. In 2008, Dr. Bloom was named associate director for science management for RML in NIAID’s Division of Intramural Research. He has also served as acting chief of the NIAID new Laboratory of Virology and acting chief of the NIAID Laboratory of Human Bacterial Pathogenesis.
Selected Publications
Ooi YS, Majzoub K, Flynn RA, Mata MA, Diep J, Li JK, van Buuren N, Rumachik N, Johnson AG, Puschnik AS, Marceau CD, Mlera L, Grabowski JM, Kirkegaard K, Bloom ME, Sarnow P, Bertozzi CR, Carette JE. An RNA-centric dissection of host complexes controlling flavivirus infection. Nat Microbiol. 2019 Dec;4(12):2369-2382.
Grabowski JM, Nilsson OR, Fischer ER, Long D, Offerdahl DK, Park Y, Scott DP, Bloom ME. Dissecting Flavivirus Biology in Salivary Gland Cultures from Fed and Unfed Ixodes scapularis (Black-Legged Tick). mBio. 2019 Jan 29;10(1):e02628-18.
Paules CI, Marston HD, Bloom ME, Fauci AS. Tickborne Diseases - Confronting a Growing Threat. N Engl J Med. 2018 Aug 23;379(8):701-703.
Offerdahl DK, Dorward DW, Hansen BT, Bloom ME. A three-dimensional comparison of tick-borne flavivirus infection in mammalian and tick cell lines. PLoS One. 2012;7(10):e47912.
Laurent-Rolle M, Boer EF, Lubick KJ, Wolfinbarger JB, Carmody AB, Rockx B, Liu W, Ashour J, Shupert WL, Holbrook MR, Barrett AD, Mason PW, Bloom ME, García-Sastre A, Khromykh AA, Best SM. The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J Virol. 2010 Apr;84(7):3503-15.
Risi GF, Bloom ME, Hoe NP, Arminio T, Carlson P, Powers T, Feldmann H, Wilson D. Preparing a community hospital to manage work-related exposures to infectious agents in BioSafety level 3 and 4 laboratories. Emerg Infect Dis. 2010 Mar;16(3):373-8.
Research Group
Investigates pathogenesis & biology of tickborne encephalitis virus complex of flaviviruses. We use in vitro, ex vivo, & animal models (mouse & tick) to examine biology of acute & persistent infection in mammalian & arthropod systems, using confocal microscopy, electron microscopy, electron cryotomography, immunohistochemistry, microarray analysis, nucleic acid sequencing, & molecular virology.

