Immunobiology and Molecular Virology Section
Established in 2019
Andrea Marzi, Ph.D.
Chief, Immunobiology and Molecular Virology Section

Major Areas of Research
- Development of filovirus animal disease models
- Identification of viral and host factors driving viral pathogenesis
- Analysis of immune responses to vaccination and challenge to identify important mediators of protection
- Development of improved prophylactic and therapeutic strategies against emerging viruses
Program Description
Highly pathogenic viruses, including filoviruses (Ebola and Marburg virus), continue to pose a significant threat to humans with their potential to cause global public health crises. In 2014, such a crisis occurred and was circumvented to become a pandemic by the global commitment of resources to fight the Ebola virus (EBOV) epidemic that devastated West Africa and spread to Nigeria, the United States, and other countries. Since 2018, frequent outbreaks of Ebola virus disease (EVD) have occurred in the Democratic Republic of the Congo.
While EBOV has been extensively studied over the past few decades, we still face gaps in our understanding of the mechanism of pathogenesis. We want to identify and fill these knowledge gaps with the goal to apply the new information to the identification of potential targets for medical intervention. Using our established capacity for in vitro and in vivo experiments in the maximum containment laboratory at RML, we are leveraging molecular approaches, including virus reverse genetics systems combined with animal models, and host transcriptome analysis, to gain insight into potential mechanisms of pathogenesis.
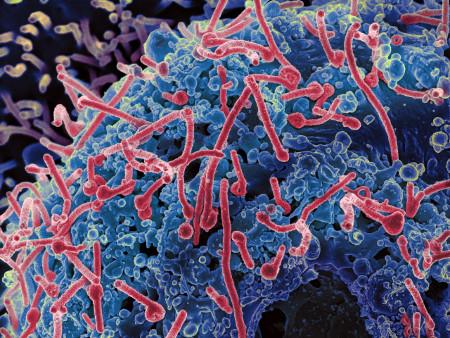
Electron micrograph Ebola virus particles (red) budding from an infected cell.
In 2019, the first EBOV vaccine was approved for human use: a live-attenuated vesicular stomatitis virus (VSV)-based vector expressing the EBOV glycoprotein (VSV-EBOV) as viral antigen. This vaccine was extensively characterized in preclinical studies at RML and deployed during the West African EBOV epidemic in a Phase 3 clinical trial, highlighting its fast-acting potential as a single-dose vaccine ideal for outbreak situations. Filoviruses are genetically distinct and divided into different genera and species, making it necessary to develop species-specific vaccines and treatments. Following the successful strategy of the VSV-EBOV, we are developing and characterizing VSV-based vaccines for other human pathogenic filoviruses, including Marburg virus and Sudan virus. Accompanying these efforts is the development of animal models for novel filoviruses like Lloviu virus in order to conduct countermeasure efficacy studies but also to evaluate the pathogenic potential of these new viruses.
Electron micrograph of vesicular stomatitis virus.
Building upon our existing expertise with the VSV vaccine platform, we used the VSV-EBOV vector as a basis for the development of live-attenuated SARS-CoV-2 vaccines when the coronavirus disease 2019 (COVID-19) pandemic started in early 2020. Because the VSV-encoded GP determines cellular tropism of the vaccine, we designed and generated vectors without (VSV-SARS2) and with the EBOV GP (VSV-SARS2-EBOV) to allow for ACE2-independent replication. We used the Syrian golden hamster model as well as rhesus macaques to evaluate the protective efficacy of vaccination with VSV-SARS2 or VSV-SARS2-EBOV.
Our long-term goal is to develop approaches that can be applied to any highly pathogenic and emerging virus threatening global public health.
Biography
Education
Dr. rer. Nat. (Ph.D.), Friedrich-Alexander University Erlangen-Nürnberg, Germany
Diplom (M.Sc.), Freidrich-Alexander University Erlangen-Nürnberg, Germany
Vordiplom (B.Sc.), Friedrich-Alexander University Erlangen-Nürnberg, Germany
Languages Spoken
GermanDr. Marzi received her Ph.D. in virology from the Friedrich-Alexander University Erlangen-Nürnberg, Germany, where she studied the glycoprotein-mediated entry of Ebola virus (EBOV) and HIV. After a short postdoc in Winnipeg, Canada, at the National Microbiology Laboratory-Public Health Agency of Canada, Dr. Marzi moved to the NIAID Rocky Mountain Laboratories in Hamilton, MT. There, she continued her BSL-4 work on vaccine development for filoviruses using primarily the vesicular stomatitis virus (VSV) platform. She conducted several significant preclinical studies for the now-approved VSV-EBOV vaccine.
The German Society of Virology recognized Dr. Marzi with the prestigious Löffler-Frosch Preis (Award) for her research on filoviruses and vaccine development in 2019. That same year, Dr. Marzi was selected as a tenure-track investigator in the NIAID Laboratory of Virology and as an NIH Distinguished Scholar. In 2023, she received tenure and is now a senior investigator in the Laboratory of Virology. Her research continues to focus on filovirus pathogenesis and animal model development, on filovirus-host interactions, as well as countermeasure development and preclinical testing for these pathogens. In response to emerging infectious disease outbreaks, her group has expanded the use of the VSV vaccine platform to other pathogens such as the influenza virus, Zika virus, and SARS-CoV-2.
Selected Publications
Fletcher P, Clancy CS, O'Donnell KL, Doratt BM, Malherbe DC, Rhoderick JF, Feldmann F, Hanley PW, Takada A, Messaoudi I, Marzi A. Pathogenic differences of cynomolgus macaques after Taï Forest virus infection depend on the viral stock propagation. PLoS Pathog. 2024 Jun 11;20(6):e1012290.
Fletcher P, O'Donnell KL, Doratt BM, Malherbe DC, Clancy CS, Rhoderick JF, Feldmann F, Hanley PW, Ksiazek TG, Geisbert TW, Messaoudi I, Marzi A. Single-dose VSV-based vaccine protects cynomolgus macaques from disease after Taï Forest virus infection. Emerg Microbes Infect. 2023 Dec;12(2):2239950.
Clancy CS, Smart G, Rhoderick JF, O'Donnell KL, Rosenke R, Schäfer A, Marzi A. Establishing a Mouse Model for Sexual Transmission and Male Reproductive Tract Persistence of Ebola virus. J Infect Dis. 2023 Apr 27:jiad118.
O'Donnell KL, Feldmann F, Kaza B, Clancy CS, Hanley PW, Fletcher P, Marzi A. Rapid protection of nonhuman primates against Marburg virus disease using a single low-dose VSV-based vaccine. EBioMedicine. 2023 Mar;89:104463.
Marzi A, Fletcher P, Feldmann F, Saturday G, Hanley PW, Feldmann H. Species-specific immunogenicity and protective efficacy of a vesicular stomatitis virus-based Sudan virus vaccine: a challenge study in macaques. Lancet Microbe. 2023 Mar;4(3):e171-e178.
Furuyama W, Shifflett K, Feldmann H, Marzi A. The Ebola virus soluble glycoprotein contributes to viral pathogenesis by activating the MAP kinase signaling pathway. PLoS Pathog. 2021 Sep 16;17(9):e1009937.
Research Group
Highly pathogenic viruses, including filoviruses, continue to pose a significant threat to humans with their potential to cause global public health crises. We are investigating host-filovirus interactions to decipher mechanisms of pathogenesis. Additionally, we develop vaccines based on vesicular stomatitis virus for emerging viral infections and conduct preclinical efficacy studies in various…


